File
advertisement

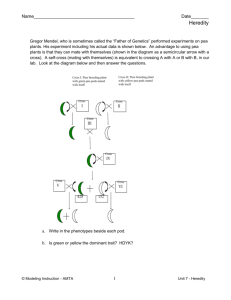
EIP Mathayom Course Description First Semester Subject: Science Grade: Mathayom 1 Periods: 4 Department: Science Course Code: SC21101 Credit: 1.5 Course Description: The course is Mathayom 1 general science, it’s a one year course designed to cover and exceed all standards and indicators. The general topics covered in the first semester with an introduction to science, the scientific method, units of measurement and general laboratory safety. The course continues with an introduction to matter, the properties of matter, followed by examination of weight, mass, volume and density. The physical and chemical changes of substances and conservation of mass are addressed. The structure of solids, liquids and gases are investigated, along with changes of state and gas laws and the periodic table are all studied. The course continues with an introduction to plants and cells, their structure and function. The importance of photosynthesis is addressed along with cell division and sexual reproduction system of flowering plants. Genetics and heredity will also be studied. Students will also be given two projects to complete throughout the semester; one project will be on environmental science; the other project will be a project related to space science. The course puts emphasis in practical application of scientific principles and explaining the world around us. The course has a textbook but much of the learning is derived from up-to-date internet resources, reflective writing, and hands-on application of the course content. The goal of the course is to provide the students with a set of personal skills that will enable them to investigate and contemplate science in a professional manner on topics they desire. EIP Mathayom Course Outline First Semester General Introduction to Science Introduction to science The scientific method Units of measurement (SI unit system) Laboratory equipment and safety Chapter 1 – Introduction to Chemistry: Introduction to Matter (pp 1 - 33) Describing matter Physical and chemical properties of matter Classifying matter – Substances, elements, atoms, molecules and compounds Mixtures Weight and mass Volume and density Physical and chemical changes of substances Conservation of mass Temperature and thermal energy Chapter 2 – Introduction to Chemistry: Solids, Liquids and Gases (pp 36 – 60) Describing a solid, liquid and gas Pressure and temperature Changes of states of matter Relationship between pressure and temperature of a gas Relationship between volume and temperature of a gas (Charles’s Law) Relationship between pressure and volume of a gas (Boyle’s Law) Chapter 3 – Introduction to Chemistry: Elements and the Periodic Table (pp68 – 118) Introduction to Atoms Organising the Elements Nonmetals and Metalloids Radioactive Elements Chapter 4 – Cells and Heredity: Introduction to cells (pp 1 – 33) Cell structure and function Chemical elements and compounds in cells Unicellular and multicellular organisms How materials move through cells by osmosis and diffusion Chapter 5 – Cells and Heredity: Cell processes and energy (pp 40 – 63) Photosynthesis – processes and importance Cellular respiration Importance of photosynthesis process of plants on living things and the environment Cell division Sexual reproduction system of flowering plants Chapter 6 – Cells and Heredity: Genetics the Science of Heredity (pp 70 - 93) What is Heredity Probability and Heredity Patterns of Inheritance Chromosomes and Inheritance EIP Mathayom Evaluation and Grading First Semester Breakdown of Grades Criteria 1st quarter 2nd quarter Total Homework Participation Behavior Labs/Group Work Tests/Quizzes 5% 5% 5% 5% 10% 5% 5% 5% 5% 10% 10% 10% 10% 10% 20% Midterm Examination Final Examination 20% 20% 20% 20% Total 50% 50% 100%