Unanticipated Event / Noncompliance Form
advertisement

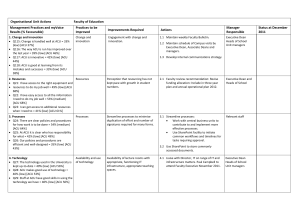
ACU IRB # ____________ Date of Approval __/__/____ Date of Expiration __/__/____ Abilene Christian University Institutional Review Board Committee & Departmental Research Review Panel Unanticipated Problem/Noncompliance Form Complete the Report and send as an e-mail attachment to orsp@acu.edu. Include any appendix materials, as applicable. ** Please see the HHS Guidance on Reviewing and Reporting Unanticipated Problems for what qualifies as requiring prompt reporting. Title of Project: Protocol #: Date of Report: Principal Investigator: Faculty Advisor (If PI is a student): Investigator Assurances Form Department / Affiliation: Phone: **Note: Faculty Advisor MUST read and sign the Degree/Credentials: Email: Address or ACU Box: Point of Contact, if other than PI (Name, phone, email): Person Reporting the Problem: Date the Problem Occurred: Date the Research Team learned of the problem**: Location where problem occurred: Participant IDs of those affected by the problem: ** Unanticipated problems that are serious UPIRSOs (Unanticipated Problems Involving Risk to Subjects or Others) should be reported within 7 days of this date, unless the UPIRSO is potentially lethal, then it should be reported within 2 days. Other unanticipated problems should be reported within 14 days of this date. What is the Nature of the Event: UPIRSOs [Complete Box 1] Protocol Deviations/Noncompliance [Complete Box 2] Other [Complete Box 3] ####_PI_EventReport_######## ACU IRB # ____________ Date of Approval __/__/____ Date of Expiration __/__/____ Box 1: UPIRSOS Please indicate the type of UPIRSO: Unexpected problem, adverse event, injury, or reaction that is definitely or probably related to the research and places participants at greater risk than previously recognized. (“Unexpected” may refer to the nature, severity, or frequency of the event) Breach of confidentiality involving private, identifiable information. Complaint from a participant which suggests an increased risk, a violation of participants’ rights or welfare, or that otherwise cannot be resolved by study staff. Other: _________________________ If an adverse event occurred, was it serious and: resulted in death was life-threatening. resulted in inpatient hospitalization or prolongation of hospitalization. resulted in persistent or significant disability/incapacity resulted in a congenital anomaly/birth defect based upon appropriate medical judgment, may jeopardize the subject’s health and may require medical or surgical intervention to prevent one of the other outcomes listed above. Box 2: Protocol Deviation or Noncompliance Please indicate the type of Noncompliance: Failure to conduct the study in accordance with all applicable regulations and policies. Failure to conduct the study in accordance with the IRB-approved protocol and which adversely affects participant safety, increases risks to participants, or violates participants’ rights and welfare. (minor deviations that do not affect safety, increase risk, or violate rights and welfare may be reported on the continuing review). Other: _________________ Box 3: Other Please indicate the type of Event: Unapproved change made to eliminate an immediate hazard. Other: ______________ ####_PI_EventReport_######## ACU IRB # ____________ Date of Approval __/__/____ Date of Expiration __/__/____ Did the problem involve any participants from a vulnerable population? Yes No Children Pregnant Women or Fetuses Neonates Decisionally Impaired Prisoners Students Other: ____________ Please describe in detail the problem being reported, including what caused it; how it may have adversely affected participant safety, increased risks to participants, or violated participants’ rights and welfare; how it was resolved; and what was the outcome: Has this issue occurred before? Yes No If yes, please indicate whether it was previously reported and provide the date of that report: Please indicate the actions planned: Action Protocol amendment (must submit an amendment form) including but not limited to changes in methods/procedures, modification of inclusion/exclusion criteria, changes to safety monitoring plan Revise Consent Form (must submit an amendment form) Notification of the problem to current and/or past participants (must submit proposed communications) Study will be voluntarily placed on hold pending resolution Study will be voluntarily closed (must submit an Inactivation Form) Additional training of research team will be provided Other ____________ No action planned ####_PI_EventReport_######## Implemented [X] Planned but not yet implemented [X] ACU IRB # ____________ Date of Approval __/__/____ Date of Expiration __/__/____ Please summarize in further detail the actions that have been implemented or are planned: Is this project being funded by an outside agency? Yes No If yes, please specify which agency: APPENDIX Identify which items are included in the appendix Signed Investigator assurance/signature form (required) Amendment Form Participant Communication Inactivation Form Other: ______________________ ####_PI_EventReport_########