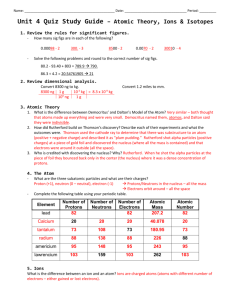
Study Guide for Chapter 4 quiz (These are not the questions on the
advertisement

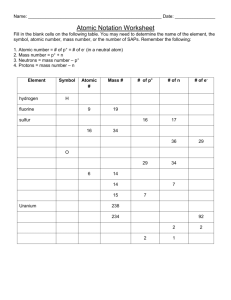
Study Guide for Chapter 4 quiz (These are not the questions on the quiz! They are simply meant to be used to study.) The charge and mass number of a proton are: The mass number of an atom is determined by The _______________ constitute(s) most of the volume of an atom. The region labeled "Y" in the diagram has a charge that is: Isotopes (such as hydrogen-1, hydrogen-2, and hydrogen-3) are atoms of the same element that differ in: The atomic number of an element is equal to: How many neutrons are there in an atom of hydrogen-3? In order for an atom to be nitrogen, it must have: Which of the following accurately summarizes the isotope Argon-40? 22 protons, 22 electrons, 18 neutrons 40 protons, 40 electrons, 18 neutrons 39.95 protons, 39.95 electrons, 21.05 neutrons 18 protons, 18 electrons, 22 neutrons An atom of uranium-238 differs from an atom of uranium-235 in the following way: Isotope Mass number Atomic number Protons Neutrons Electrons lead-_____ 127 How many electrons would be found in an atom of oxygen (atomic number 8)? Find the average atomic mass with the given isotopes: 55Fe 15% and 56Fe 85% An electron emitted from the nucleus during some kinds of radioactive decay is known as: For the most common types of radioactive decay, the order of mass from lightest to heaviest is: An alpha () particle is essentially a ____________________ nucleus. _____+ 10n → 14256Ba + 9136Kr + 310n 27 13Al + 42He → 3015P + ______