Donor CMV
advertisement
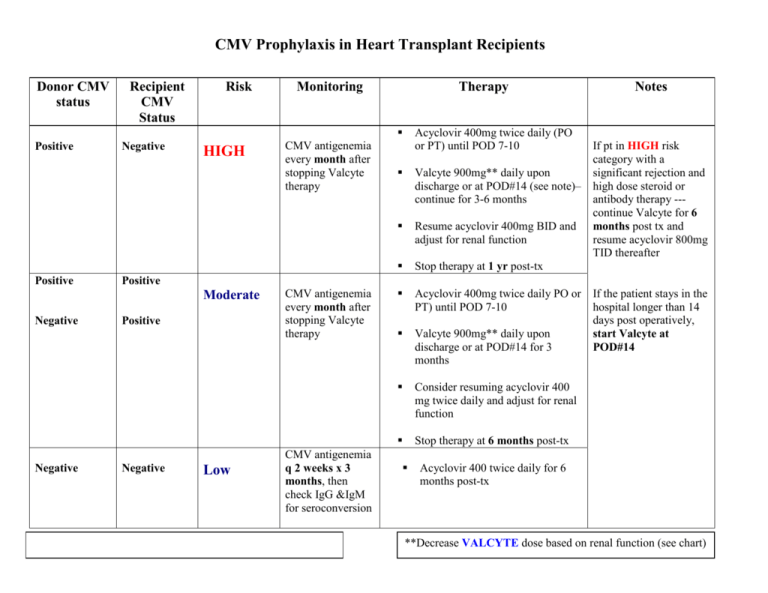
CMV Prophylaxis in Heart Transplant Recipients Donor CMV status Recipient CMV Status Risk Monitoring Therapy Positive Positive Negative HIGH Negative Acyclovir 400mg twice daily (PO or PT) until POD 7-10 Valcyte 900mg** daily upon discharge or at POD#14 (see note)– continue for 3-6 months Resume acyclovir 400mg BID and adjust for renal function Stop therapy at 1 yr post-tx Acyclovir 400mg twice daily PO or PT) until POD 7-10 Valcyte 900mg** daily upon discharge or at POD#14 for 3 months Consider resuming acyclovir 400 mg twice daily and adjust for renal function Stop therapy at 6 months post-tx If pt in HIGH risk category with a significant rejection and high dose steroid or antibody therapy --continue Valcyte for 6 months post tx and resume acyclovir 800mg TID thereafter Positive Moderate Negative CMV antigenemia every month after stopping Valcyte therapy Notes Positive Negative Low CMV antigenemia every month after stopping Valcyte therapy CMV antigenemia q 2 weeks x 3 months, then check IgG &IgM for seroconversion If the patient stays in the hospital longer than 14 days post operatively, start Valcyte at POD#14 Acyclovir 400 twice daily for 6 months post-tx **Decrease VALCYTE dose based on renal function (see chart) Renal Dosing of Valcyte (Valganciclovir) Induction/ Treatment dose Prophylaxis/ Maintenance Dose CrCl ≥ 60ml/min 900 mg twice daily 900 mg daily (or 450 twice daily) 900 mg once daily (or 450 twice daily) 450 mg daily 450 mg daily 450 mg every other day 450 mg every other day 450 mg twice weekly CrCl 69-40 ml/min CrCl 39-25 ml/min CrCl 25-10 ml/min References: Akalin JS, Bronberg V, Shegal S, et al. Six months valganciclovir prophylaxis significantly decreased cytomegalovirus infection incidence in thymoglobulin treated patients. Am J Transplant 2004(4):495. Valcyte [package insert]. Philadelphia, PA: Wyeth; September 2007. Kalil AC, Levitsky J, Lyden E, et al. Meta Analysis: the efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med 2005; 143: 870-80. Cvetkovic RS, Wellington K. Valganciclovir: A review of its use in the management of CMV infection and disease in immunocompromised patients. Drugs 2005; 65(6):859-78. Kusne S, Shapiro R, Fung J, et al. Prevention and treatment of cytomegalovirus infection in organ transplant recipients. Transpl Infect Dis. 1999;1:187-203. Singh N. Preemptive therapy versus universal prophylaxis with Ganciclovir for CMV in solid organ transplant recipients. Clin Infect Dis. 2001; 32: 742-51. Khoury JA, Storch GA, Bohl DL, et al. Prophylactic versus preemptive oral valganciclovir for the management of CMV infection in adult renal transplant recipients. Am J Transplantation. 2006; 6:2134-2143. Singh N. Cytomegalovirus infection in solid organ transplant recipients: new challenges and their implications for preventive strategies. J Clin Virol. 2006; 35 (4):474-44. Revised 9/2007