Excercieses 3
advertisement
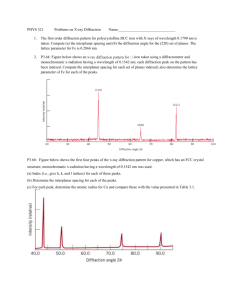
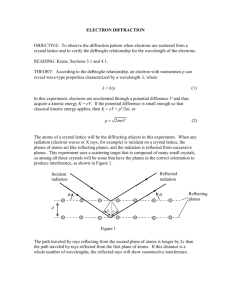
Solid State Physics (1), Phys3710 EX.3 1. For BCC iron, compute (a) the interplanar spacing, and (b) the diffraction angle for the (220) set of planes. The lattice parameter for Fe is 0.2866 nm. Also, assume that monochromatic radiation having a wavelength of 0.1790 nm is used, and the order of reflection is 1. Solution: (a) The value of the interplanar spacing is determined, with a= 0.2866 nm, and h= 2, k = 2, l = 0, and since we are considering the (220) planes. Therefore, (b) The value of order reflection: ɵ may now be computed, with n=1 since this is a first- The diffraction angle is 2ɵ or: 1 2. The metal rhodium has an FCC crystal structure. If the angle of diffraction for the (311) set of planes occurs at 36.12o (first-order reflection) when monochromatic x-radiation having a wavelength of 0.0711 nm is used, compute (a) the interplanar spacing for this set of planes, and (b) the atomic radius for a rhodium atom. Solution: (a) From the data given in the problem, and realizing that 36.12° = 2θ, the interplanar spacing for the (311) set of planes for rhodium may be computed using the following Equation: (b) In order to compute the atomic radius we must first determine the lattice parameter, a, and then R , since Rh has an FCC crystal structure. Therefore, 2 3. The metal niobium has a BCC crystal structure. If the angle of diffraction for the (211) set of planes occurs at 75.99o (first-order reflection) when monochromatic x-radiation having a wavelength of 0.1659 nm is used, compute (a) the interplanar spacing for this set of planes, and (b) the atomic radius for the niobium atom. Solution: (a) From the data given in the problem, and realizing that 75.99° = 2θ, the interplanar spacing for the (211) set of planes for Nb may be computed using the following Equation: (b) In order to compute the atomic radius we must first determine the lattice parameter, a, and then R since Nb has a BCC crystal structure. Therefore, 3