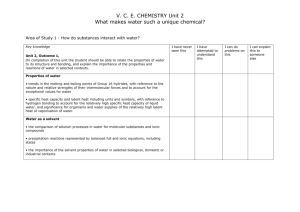
The Natural Science Lines I and II
advertisement

The Natural Science Lines I and II General Chemistry 1st year 3 periods per week Textbook: Chemistry; The Central Science by T.L. Brown, H.E. LeMay Jr., B.E. Bursten, and C.J Murphy, Chapters: 1 – 4 Ch. 1 Matter and Measurements Classification and properties of matter, SI and derived SI units, precision and accuracy, significant figures in calculations, dimensional analysis. Ch. 2 Atoms, molecules, and ions Atomic theory, atomic and mass numbers, isotopes, atomic and average atomic masses, molecular and empirical formulas, predicting ionic charges and ionic compounds, nomenclature of ionic and binary molecular compounds, alkanes and some simple derivatives of alkanes. Ch. 3 Calculations with Chemical Formulas and Equations Balancing equations, states of reactants and products, formula and molecular weights, mole, molar mass, interconverting mass, moles and number of particles. Empirical formulas from elemental analysis, stoichiometry and quantitative information from balanced equations, limiting reactants and theoretical yields. Ch. 4 Aqueous Reactions and Solution Stoichiometry General properties of aqueous solutions: electrolytic properties, ionic and molecular compounds in water, strong and weak electrolytes. Precipitation reactions: Solubility guidelines for ionic compounds, metathesis reactions, ionic equations. Acid-base reactions: strong and weak acids and bases, neutralization reactions and salt formation. Oxidation-reduction reactions: oxidation numbers, oxidation of metals by acids and metal salts, activity series. Concentration of solutions: molarity, interconverting molarity, moles and volume. Dilution, solution stoichiometry and chemical analysis by titrations. General Chemistry 2nd year Lectures: 3x40 min. per week Textbook: Chemistry; The Central Science By T.L. Brown, H.E. LeMay Jr., B.E. Bursten, and C.J Murphy Chapters: 5-11 Ch. 5 Thermochemistry Nature and units of energy, the first law of thermodynamics, relating internal energy change to heat and work, endo- and exothermic processes, enthalpy and enthalpies of reactions, calorimetry, Hess´s law, enthalpies of formation. Ch. 6 Electronic Structure of Atoms The wave and particle nature of light, photon energy, line spectra and Bohr hydrogen model, quantum mechanics and atomic orbitals, electron configuration of many-electron atoms, electron configuration and the Periodic table. Ch. 7 Periodic Properties of the Elements Effective nuclear charge and trends in properties: sizes of atoms and ions, ionic energy, electron affinities. Metals, nonmetals and metalloids, group trends in alkali and earth alkali metals, oxygen group, halogens and nobel gases. Ch. 8 Basic Concepts of Chemical Bonding Ionic and covalent bonds, octet rule, bond polarity and electronegativity, Lewis structures, formal charges, resonance structures, exceptions to the octet rule, bond enthalpies and enthalpies of reactions. Ch. 9 Molecular Geometry and Bonding Theories Molecular shapes and the VSEPR model, molecular polarity, a short introduction to covalent bonding and orbital overlap. Ch. 10 Gases Pressure, barometer, the gas laws of Boyle, Charles and Avogadro. The ideal-gas equation and its application in chemical reactions, gas mixtures and partial pressures, kineticmolecular theory. Ch. 11 Intermolecular Forces, Liquids and Solids A molecular comparison of gases, liquids and solids. Intermolecular forces: ion-dipole, dipole-dipole, London dispersion, hydrogen bonding, comparison of intermolecular forces, viscosity and surface tension of liquids. Vapor pressure: effect of intermolecular forces, temperature dependence, boiling point. Structure of solids: simple, body-centered and face-centered cubic unit cells, NaCl(s)structure. Bonding in solids: molecular, covalent-network, ionic and metallic solids. Ch. 25 The Chemistry of Life: Organic and Biological Chemistry Hybridizations, sp3-, sp2- and sp3-orbitals, - and - bonds, structural isomers, nomenclature, structural and physical properties of: alkanes, cycloalkanes, and alkenes. Laboratory experiments: 2x40 min. every other week The chemistry experiments are largely bases on: Chemistry with Computers using Logger ProTM and Vernier Sensors, by D.D. Holmquist and D. L. Volz 1. Formation and reactions of carbon dioxide. 2. Planning and preparations of solutions. 3. Endo- and exothermic reactions. 4. Additivity of heats of reaction: Hess´s Law 5. Analysis of FeSO4•nH2O by permanganate titration. 6. Determination of the stoichiometry of the iodometric reaction. 7. Conductivity of solutions: The effect of concentration. 8. Determining the concentration of a solution: Beer´s Law. 9. Pressure-temperature relationship of a gas. 10. Reaction of magnesium in hydrochloric acid: collecting and determining the amount of a hydrogen gas. 11. Evaporation and intermolecular forces. 12. Structures and nomenclature of organic molecules: an exercise using the program ChemSketch. General Chemistry 3rd year 3 periods per week Textbook: Chemistry; The Central Science By T.L. Brown, H.E. LeMay Jr., B.E. Bursten, and C.J. Murphy Chapters: 14-17 and 19-20 Ch. 15 Chemical Equilibrium The law of equilibrium, equilibrium constants Kc and Kp, heterogeneous equilibria, calculating equilibrium constants. Application of equilibrium constants: reaction quotient and direction of a reaction, calculating equilibrium concentrations. Le Chatelier principle: effect of change in concentrations, volume, pressure and temperature. The effect of catalysts. Ch. 16 Acid-Base Equilibria Brönsted-Lowry acids-base definition, autoionization of water, pH scale, strong and weak acids and bases, calculating pH of weak acid and weak base solutions and solutions of their salts, polyprotic acids, acid-base properties of salt solutions, acid-base behavior and chemical structure, Lewis acids and bases. Ch. 17 Additional Aspect of Aqueous Equilibria Common-ion effect, buffered solution, calculating the pH-curve for a acid-base titration: strong acid–strong base, weak acid-strong base, weak base-strong acid. Solubility equilibria, solubility product constant (Ksp), solubility (g/L) and molar solubility. The effect of common-ion, pH and complex formation on solubility. Precipitation and separation of ions. Ch. 14 Chemical Kinetics Reaction rate, determining a rate law from initial rates, temperature and rate, collision model, orientation factor and activation energy, Arrhenius equation, determining activation energy. Reaction mechanism: elementary reactions and their rate laws, rate-determining step, deriving a rate law for few simple multistep mechanism. Catalysis: homogenous and heterogenous catalysis, enzymes. Ch. 19 Chemical Thermodynamics Spontaneous processes, entropy and the second law of thermodynamics, a short introduction to the molecular interpretation of entropy, entropy change in chemical reactions, Gibbs free energy, free energy and temperature, free energy and the equilibrium constant. Ch. 20 Electrochemistry Balancing oxidation-reduction reactions, voltaic cells, cell electromotive force (EMF), free energy and EMF, Nernst equation, concentration cells, batteries, corrosion, electrolysis. Laboratory experiments: 2x40 min. every other week The chemistry experiments are largely bases on: Chemistry with Computers using Logger ProTM and Vernier Sensors, by D.D. Holmquist and D. L. Volz 1. Using conductivity to find the equivalence point for the reaction between saturated Ba(OH)2 and H2SO4. 2. Colorimetric determination of Kc for: Fe3+ + SCN- FeSCN2+. 3. The temperature dependence of the vapor pressure of methanol. 4. Titration curve of a weak acid measured with a computer-interfaced pH electrode. 5. Titration curve of a Na2A salt measured with a computer-interfaced pH electrode. 6. Separation of a mixture of Ni2+, Co2+ and Fe3+ with an ion-exchange column. 7. Determination of the composition of Cu(NH3)n2+ complex by HCl titration of aqueous and chloroform solutions. 8. Determination of the rate law of: S2O82- + 2I- 2SO42- + I2 9. Electrochemical cell: establishing a table of reduction potential and application of Nernst equation. 10. Electrolysis of potassium iodide solution: identification of products and halfreactions. 11. EDTA-titration analysis of calcium in milk. 12. Determination of molecular weight by freezing-point depression. Organic Chemistry and Biochemistry 3rd year 3 periods per week Textbook: Fundamentals of Organic Chemistry, by J. McMurry and E. Simanek Chapters: 1-10, 12 and 14-17 Ch. 1 Structure and Bonding Chemical bonding, covalent bonds, hybridization, sp3-, sp2- and sp3-orbitals, - and bonds, polar bonds. Ch. 2 The Nature of Organic Molecules Alkanes, alkyl groups, isomers, naming branched-chain alkanes, conformations, cycloalkanes, cis-trans isomerism of cycloalkanes, chair conformation of cyclohexane. Ch. 3 The Nature of Organic Reactions: Alkenes Naming alkenes, cis-trans isomers of alkenes, kinds of organic reactions: addition, elimination, substitution, rearrangement, mechanism of HCl addition to ethylene. Ch. 4Reactions of Alkenes and Alkynes Addition reactions of alkenes: hydro-halogenation, hydration, halogenation and hydrogenation, Markovnikov´s rule, carbocation structure and stability, anti and syn stereochemistry, 1,4-addition of conjugated dienes, naming alkynes. Ch. 5 Aromatic Compounds Resonance structure, naming aromatic compounds, electrophilic aromatic substitution reactions: bromination Ch. 6 Stereochemistry Enantiomers, chirality, chiral centers, optical activity, chirality in nature. Ch. 7 Alkyl Halides Naming and preparing alkyl halides, reaction of alkyl halides, Grignard reagents. SN2 and SN1 reactions: rate, mechanism, stereochemistry, steric effects, leaving groups. Ch. 8 Alcohols, Phenols, and Ethers Naming alcohols, properties of alcohols, synthesis of alcohols, reactions of alcohols: dehydration and oxidation. Ch. 9 Aldehydes and ketons Chemistry of the carbonyl group, naming aldehydes and ketons. Ch. 10 Carboxylic Acids and Derivatives Naming carboxylic acids and derivatives, properties of carboxylic acids and derivatives, acidity of carboxylic acids, synthesis of carboxylic acids, nucleophilic acyl substitution reactions. Ch. 12 Amines Naming amines, structure and properties of amines. Ch. 14 Biomolecules: Carbohydrates Classification of carbohydrates, aldoses and ketoses, configuration of monosaccharides, Fischer projections, D- and L-sugars, configuration of aldoses, cyclic structures of monosaccharides, hemiacetal formation, disaccharides, glycosidic bond, 1,4-link, maltose, cellobiose, sucrose, polysaccharides, cellulose, starch, glycogen. Ch. 15 Biomolecules: Amino Acids, Peptides, and Proteins Structure of amino acids, peptide synthesis in the laboratory, the peptide bond, classification of proteins, protein structure: primary, secondary, tertiary and quaternary structure. keratin, -helix, fibroin, -pleated sheet. Enzymes Ch. 16 Biomolecules: Lipids and Nuclear Acids Lipids, fats and oils, triacylglycerols, saturated and unsaturated fatty acids, soaps, stereoids. Structures of nucleotides, structure of nucleic acids: DNA. Ch. 17 The Organic Chemistry of Metabolic Pathways ATP, catabolism of fats, -oxidation pathway, catabolism of carbohydrates, glycolysis, the citric acid cycle. Laboratory experiments: 2 periods every other week 1. Constructing structures of organic molecules: an excersise using molecular models. 2. Paper chromatographic separation of the dyes of marker pens. 3. Analysis of vitamin C. 4. Identification of common functional groups. 5. Preparation of a face cream: an investigation of its essential components. 6. Synthesis of wintergreen and aspirine. 7. Preparation and purification of acetanilide. 8. Synthesis of nylon and preparation of rubber from latex. 9. Isolation of casein and lactose from milk. 10. Saponification. -------------