DOC
advertisement

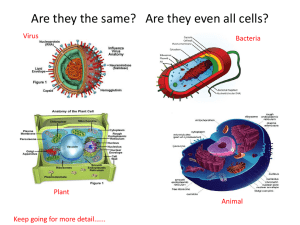
Cell counting using a hemacytometer 1. Mix 10 l of cells with 10 l of 0.4 % Trypan Blue (Sigma T-8154 100ml) in an 1.5ml centrifuge tube (1:1 dilution) 2. Remove the hemacytometer and the glass coverslip from the petridish containing ethanol 3. Dry the hemacytometer and the coverslip using Kimwipes 4. Place coverslip on top of hemacytometer and pipette 10 l of cells into the sample groove 5. Count at least 2 large squares (shown above) and average. Remember to count both dead cells (blue) and live cells (clear) 6. Cell density = Average * 2 * 104 Notes a. Since we mixed 10 l of cells with 10 l of dye we used 2 in the formula above. If you use a different dilution use the appropriate correction. E.g. if you mix 2 l of cells with 18 l of trypan blue and loaded 10 ml of the mixture your formula will be Avg*10*104 b. You should have to count no more than 80 cells in one of these large squares. If there are too many cells dilute appropriately c. Do not wait more than 15min after mixing cells with Trypan blue. 7. Use a calculator or an online program like http://www.changbioscience.com/cell/hemo.html to calculate both cell density and viability. 8. Thoroughly wash both hemacytometer and coverslip with ethanol and return to petridish.