Chapter5Part1Transcript
advertisement

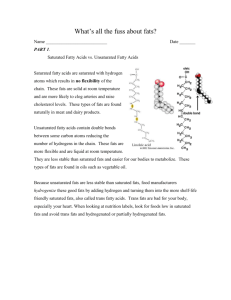
BIOL 117, Nutrition, Chapter 5, Part 1 Note: please transcribe from the mp4 to this file Chapter 3 only. Please write where you were at in the video (the time) when you finished transcribing. It is also helpful to include the slide number. Thank you! Slide 1-Chapter 5, Part 1, Lipids First of all, what are lipids? Well, lipids are another class of organic molecules. We just talked in chapter 4 about carbohydrates. They are also organic molecules; and then we’ll talk about proteins in chapter 6another type of organic molecule. Basically what that means is that have to have a carbon and hydrogen skeleton and they’re a fairly large molecule as compared to some of the other molecules we’ll talk about, like vitamins and minerals. Lipids dissolve very well in alcohol, ether, or acetone, and not as well in water. So we say they’re less soluble in water. We can also describe lipids as being hydrophobic, which technically means “water fearing,” or lipophilic which means “fat loving.” So basically lipid molecules are attracted to other lipid molecules, but they’re not attracted very easily to molecules like water. We’ll be looking at three major classes of lipids: triglycerides, phospholipids, and sterols. Make sure that you understand the three main classes, and then of course any details that we go into for each. Slide 2 We can start with fatty acids. Fatty acids are the basic building block of lipids. So the way that different fatty acids are put together makes up a very unique type of lipid in each case. So the basic structure-we can look at the diagram here, there’s a carboxyl group and that’s COOH and that’s found at the alpha end of the fatty acid. Alpha and omega is just a way to identify which end of the molecule you’re talking about, so don’t get too tripped up with that. There’s a methyl group (CH3) and that’s at the other end, the omega end. And you can see that there are carbons in between as well, so the length of the carbon in between can vary. Right now, you see 1,2, 3, 4,5, 6 carbon molecules not including the carboxyl group and the methyl group. Slide 3 The chain length tells you a lot about the lipid molecule. It can range, there can be anywhere from 4 to 24 carbons. If a lipid has a shorter chain, that means that it is actually more water soluble, meaning it’s easier to break those bonds and it can dissolve in water a little more easily. It would also have a lower melting point. And what that means is that it would be more liquefied. If we could talk about looking at something at room temperature, the shorter the chain length, the more liquid it would be. Longer chains have a higher melting point. So this is something that at regular room temperature would be a solid. So something like butter would technically be a solid at room temperature and something like olive oil would naturally be a liquid at room temperature. So olive oil compared to butter would have less of a carbon chain. So, the longer the chain, the less soluble it is in water. This is important because we’re also talking about how soluble these things are in our bodies and not just water. If you were to put it in a jar of water, cup of water, but how it would act in our bodies and how we would break them down. Slide 4 So figure 5.3 just kind of reminds you of the chain length. Again, the longer the chain length, the more solid something is at room temperature; and the more likely it is to, it’ll have a harder time probably getting through your body, as far as being broken up. Slide 5 So this leads us to talking about saturation. If we look at a carbon atom, if we were to look at the chemistry of it, you would find that is has 4 available bonds. So what that means is that it’s looking for other atoms to bond to it and those bonds fill up with either carbons or other hydrogens or other oxygens. We say that a fatty acid is saturated if all of the open sides are of those 4 carbon sides, if we have several hydrogen atoms bound to any sites that are open. You’ll always see that there’s another carbon bound to another carbon but then there’s three other possible spaces for hydrogen atoms to bond. So in the case of saturated, all the carbon atoms would have single bonds as compared to double bonds. If a carbon is bonded to another carbon with a single bond that means that the other bonds are available for other things and they would be more saturated. Unsaturated fatty acids, it just means that the carbons have at least one double bond. The more unsaturated it is, the larger amount of double bonds that the molecule will have. So having double bonds actually is better, health-wise, because it’s unsaturated; there’s less hydrogen atoms involved. And again, it’s easier for the body to break down. Slide 6 This model here just shows you the structure of a carbon atom. There are actually 6 electrons, and we’ll find 2 in the first orbital, 4 in the second orbital, and the second orbital would like to acquire 4 more electrons in order to be stable. So essentially what this is saying is that carbon is looking for 4 other atoms to bond with. Sometimes this is another carbon molecule, itself; sometimes it’s a hydrogen, or possibly an oxygen, depending upon where you’re at in the chain. You don’t need to worry about the chemistry too much here, but just realize that carbon has 4 available opportunities for bonding. Slide 7 When we look at a diagram like this, you can compare saturated fat to monounsaturated, to polyunsaturated. So you’ll notice in the top diagram, we have carbons. If you look in the middle here, you don’t have to look at the carboxyl group or the methyl group, but if you look in the middle, you see that all those carbons have a hydrogen bonded to them, if there not bonded to another carbon. So, you’ll see a lot of hydrogen atoms there. The next case, the monounsaturated, you’ll see right there in the middle two carbon molecules have a double bond; so they have a double covalent bond. Therefore, that’s one less hydrogen that’s involved. If you look further down, a polyunsaturated lipid has 2 or more double bonds as well. So the more double carbon bonds, the better. Slide 8 So, when we look at foods, they’re really a mixture. If you look at food labels, you’ll see that you have some unsaturated fats and you may have some saturated fats, as well. So again we can kind of look at what a food looks like at room temperature. A food that has more unsaturated fatty acids than saturated will have a lower melting point, meaning it melts easier. It would be a liquid at room temperature. And, as I talked about before, an example is olive oil and then also soybean oil. A food that has more saturated fatty acids than unsaturated would have a higher melting point. This means it would be a solid at room temperature and things that fall into this category: chocolate, meats, butter; of course there are other molecules involved here, but essentially the fats in there make those solid. Slide 9 Another aspect we can look at here are Cis versus trans fatty acids. You’ve probably heard of trans fats and that refers to what I’m going to talk about next. This has to do with the carbon chain. So in the Cis version of a fatty acid, there are 2 hydrogens that are on the same side of the chain. This makes the fatty acid chain have a little bend in it and it’s less stable. It will actually spoil or turn rancid by oxidation. So if this type of food had Cis fatty acids and it were exposed to oxygen, it would spoil faster. Trans, on the other hand, The H-the hydrogens are on opposite sides. This leads to a straighter chain and it makes the molecule more stable; it makes the fatty acid more stable. If it’s more stable, it’s less likely to be broken down as easily and it lasts longer. So sometimes what happens is companies have found a way to actually add even more hydrogens to a fatty acid. If you can increase the amount of hydrogens, then you increase the shelf life because you’ll make it a more stable molecule. Unfortunately, this also is harder for your body to break down, so it contributes to your bad cholesterol, your LDL cholesterol-which we’ll talk about in a little bit, and that leads to a higher risk of heart disease. So what this means is that if you’re looking at a food product, you should always look at the labels and if you see that a label says that it has hydrogenated fats added, that’s going to have trans fats in them and that’s about the worst fat that you can have. So if you have 2 products that you were willing to buy, and one does not have hydrogenated fats, but the other one does, you should choose the one that does not. It may not last as long in your cupboard, but it will be healthier for you. And some of these products can last for months or even a year because they have this hydrogenation added to them. So, think of them lasting in your cupboard, but also think of them lasting in your arteries, as well, as you make your choice. Slide 10 Stopped at 09:54