Stiff person syndrome
advertisement
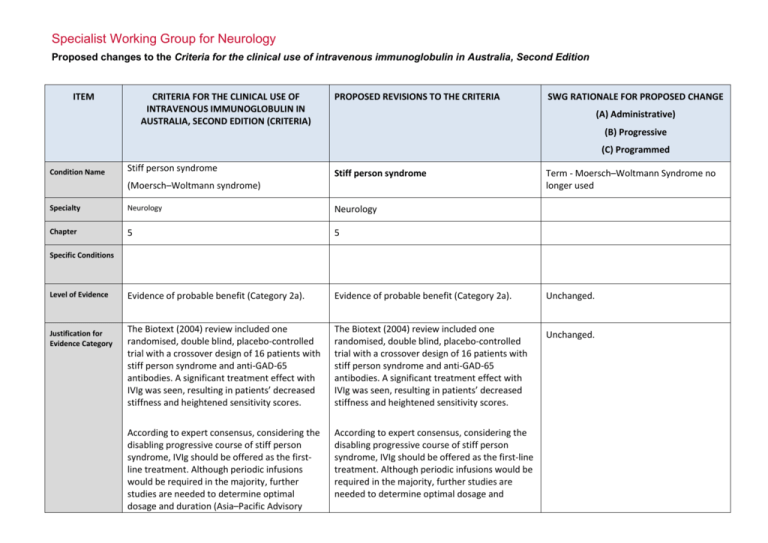
Specialist Working Group for Neurology Proposed changes to the Criteria for the clinical use of intravenous immunoglobulin in Australia, Second Edition ITEM CRITERIA FOR THE CLINICAL USE OF INTRAVENOUS IMMUNOGLOBULIN IN AUSTRALIA, SECOND EDITION (CRITERIA) PROPOSED REVISIONS TO THE CRITERIA SWG RATIONALE FOR PROPOSED CHANGE (A) Administrative) (B) Progressive (C) Programmed Condition Name Stiff person syndrome Stiff person syndrome (Moersch–Woltmann syndrome) Specialty Neurology Neurology Chapter 5 5 Evidence of probable benefit (Category 2a). Evidence of probable benefit (Category 2a). The Biotext (2004) review included one randomised, double blind, placebo-controlled trial with a crossover design of 16 patients with stiff person syndrome and anti-GAD-65 antibodies. A significant treatment effect with IVIg was seen, resulting in patients’ decreased stiffness and heightened sensitivity scores. The Biotext (2004) review included one randomised, double blind, placebo-controlled trial with a crossover design of 16 patients with stiff person syndrome and anti-GAD-65 antibodies. A significant treatment effect with IVIg was seen, resulting in patients’ decreased stiffness and heightened sensitivity scores. According to expert consensus, considering the disabling progressive course of stiff person syndrome, IVIg should be offered as the firstline treatment. Although periodic infusions would be required in the majority, further studies are needed to determine optimal dosage and duration (Asia–Pacific Advisory According to expert consensus, considering the disabling progressive course of stiff person syndrome, IVIg should be offered as the first-line treatment. Although periodic infusions would be required in the majority, further studies are needed to determine optimal dosage and Term - Moersch–Woltmann Syndrome no longer used Specific Conditions Level of Evidence Justification for Evidence Category Unchanged. Unchanged. Description and Diagnostic Criteria Board 2004). duration (Asia–Pacific Advisory Board 2004). Patients with stiff person syndrome present with symptoms related to muscular rigidity and superimposed episodic spasms. The rigidity insidiously spreads involving axial muscles, primarily abdominal and thoracolumbar, as well as proximal limb muscles. Typically, cocontraction of truncal agonist and antagonistic muscles leads to a board-like appearance with hyperlordosis. Less frequently, respiratory muscle involvement leads to breathing difficulty and facial muscle involvement to a mask-like face. Patients with stiff person syndrome present with symptoms related to muscular rigidity and superimposed episodic spasms. The rigidity insidiously spreads involving axial muscles, primarily abdominal and thoracolumbar, as well as proximal limb muscles. Typically, cocontraction of truncal agonist and antagonistic muscles leads to a board-like appearance with hyperlordosis. Less frequently, respiratory muscle involvement leads to breathing difficulty and facial muscle involvement to a mask-like face. Investigations that may be useful for diagnosis include auto-antibodies to GAD-65 or GAD-67, electromyography recordings from stiff muscles that may show continuous discharges of motor unit, and cerebrospinal fluid oligoclonal bands. Investigations that may be useful for diagnosis include auto-antibodies to GAD-65 or GAD-67, electromyography recordings from stiff muscles that may show continuous discharges of motor unit, and cerebrospinal fluid oligoclonal bands. Diagnosis is required Yes By which speciality Diagnosis must be verified No By which speciality Neurologist Tighter control of diagnosis – limited to Neurologist rather than being verified by a Neurologist (B) SWG decision that diagnostician and treating specialist should be a neurologist. This is a very rare condition. Formal neurological assessment tests have been defined to confirm eligibility and clinical response. Exclusion Criteria Indications Treatment of significant functional impairment in patients who have a verified diagnosis of stiff person syndrome. Stiff Person Syndrome or variants with significant disability Change Revise ‘impairment’ to ‘disability’ and to include variants. Rationale Reworded for consistency with other National Blood Authority pg. 2 indications - modifying language to ‘disability’ and appropriately indicating that variants are eligible for treatment Qualifying Criteria Significant functional impairment in patients who have a verified diagnosis of stiff person syndrome made by a neurologist. Stiff Person Syndrome or variants with significant disability Neurologist confirmed diagnosis of Stiff Person Syndrome or its variants with significant disability as measured by the Modified Rankin Functional ADL Score (of at least 4 points) and the Distribution of Stiffness Index (of at least one point). The Ig governance program requires formal eligibility criteria with supporting evidence to be defined for each condition. Eligibility is to be confirmed by Modified Rankin and Distribution of Stiffness Index because these methods have been demonstrated as effective through Randomised Controlled Trials. References: Rankin J. “Cerebral vascular accidents in patients over the age of 60.” Scott Med J 1957;2:200-15 Bonita R, Beaglehole R. “Modification of Rankin Scale: Recovery of motor function after stroke.” Stroke 1988 Dec;19(12):1497-1500 Van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. “Interobserver agreement for the assessment of handicap in stroke patients. Original version of Modified Rankin has been shortened to display more easily in the list of values. Review Criteria National Blood Authority Review Regular review by a neurologist is required; frequency as determined by clinical status of patient. For stable patients on maintenance treatment, review by a neurologist is required at least annually. Stiff Person Syndrome or variants with significant disability Effectiveness Objective indicators of relief of symptoms of Clinical documentation of effectiveness is necessary for continuation of IVIg therapy. IVIg should be used for six months before determining whether the patient has responded. Review by a neurologist is required after six months and at least annually thereafter. pg. 3 stiffness, including: •improvement or stabilisation of activities of daily living scores; •other specialised scoring systems, such as distribution-of-stiffness index and heightened sensitivity scale. Effectiveness can be demonstrated by objective findings of improvement in symptoms of stiffness. On review of an initial authorisation period Patient demonstrates relief of symptoms of stiffness and disability as demonstrated by a Functional Assessment ADL - Modified Rankin Score and a Distribution of Stiffness Index Score (greater than the qualifying scores). On review of a continuing authorisation period Dose Induction: 2 g/kg in 2 to 5 divided doses. Maintenance: 1–2 g/kg, 4–6 weekly. Aim for the minimum dose to maintain optimal functional status. Dosing above 1 g/kg per day is contraindicated for some IVIg products. Refer to the current product information sheet for further information. The aim should be to use the lowest dose possible that achieves the appropriate clinical National Blood Authority Patient demonstrates stable symptoms of stiffness demonstrated by a Functional ADL Score – Modified Rankin Score (greater than or equal to the scores at the last review and greater than the qualifying scores). Stiff person Syndrome with significant functional impairment There will be a lower limit defined as 1g for induction dose. Induction Dose: Up to 2 g/kg in 2 to 5 divided doses Maintenance While maximum dosing is unchanged, prescribers are encouraged to titrate the dose to the individual’s response and supported in giving divided doses more frequently than monthly. Maintenance Dose: 0.4–1 g/kg, 2–6 weekly. The amount per dose should be titrated to the individual’s response. A maximum dose of 2 g/Kg may be given in any 4 week period. This might be by divided doses more frequently than monthly. The aim should be to use the lowest dose pg. 4 outcome for each patient possible that achieves the appropriate clinical outcome for each patient. Dosing above 1 g/kg per day is contraindicated for some IVIg products. Refer to the current product information sheet for further information. BIBLIOGRAPHY Biotext 2004, ‘Summary data on conditions and papers’, in A systematic literature review and report on the efficacy of intravenous immunoglobulin therapy and its risks, commissioned by the National Blood Authority on behalf of all Australian Governments, pp. 190–1. Available from: www.nba.gov.au/pubs.htm [cited 7 Dec 2007] Dalakas, MC 2005, ‘The role of IVIg in the treatment of patients with stiff person syndrome and other neurological diseases associated with anti-GAD antibodies’, Journal of Neurology, vol. 252, suppl. 1, pp. 119–25. Dalakas, MC, Fujii, M, Li, M, et al 2001, ‘High-dose intravenous immune globulin for stiff-person syndrome’ [see comment], New England Journal of Medicine, vol. 345, no. 26, pp. 1870–6. Kornberg, AJ, for the Asia–Pacific IVIg Advisory Board 2004, Bringing consensus to the use of IVIg in neurology. Expert consensus statements on the use of IVIg in neurology, 1st edn, Asia–Pacific IVIg Advisory Board, Melbourne, pp. 70–2. Rowland, LP & Layzer RB 2005, ‘Stiff man syndrome (Moersch– Woltman syndrome)‘, in LP Rowland (ed.), Merritt’s Neurology, 11th edn, Lippincott Williams & Wilkins, Philadelphia, p. 927. END OF DOCUMENT National Blood Authority pg. 5