14.8 Acid-Base Titration
advertisement

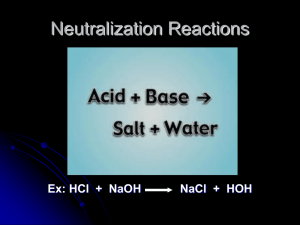
Name ____________________________________________ Date _________________________ Period__ Sample Problems for Chapter 14: Acids and Bases 14.7 Reactions of Acids and Bases (Read pgs. 465- 468 in the chemistry textbook) 14.8 Acid-Base Titration (Read pgs. 468 - 470 in the chemistry textbook) 1. (a) What is the definition of a salt? (b) Here are some examples of making salts: (i) from acids: take away the H+ and replace it with a cation Acid Salt Formula: Formula: (ii) from bases: take away the OH─ and replace it with an anion Base Formula: 2. Salt Formula: (a) What is a neutralization reactions: (b) What 2 products are formed during a neutralization reaction? 1 3. Write the following equations showing the formation of a salt from the reaction of a metal with and an acid: (a) aqueous sodium hydroxide + hydrochloric acid → water + aqueous sodium chloride (b) nitric acid and aqueous barium hydroxide → water + aqueous barium nitrate (c) solid magnesium hydroxide + hydrobromic acid → aqueous magnesium bromide + water (d) solid zinc hydroxide l + nitric acid → aqueous zinc nitrate + water 4. Predict the products of the following neutralization reactions. Use a solubility chart to determine the phase of matter of the salt. Balance the resulting equation. (a) H2SO4(aq) + Mg(OH)2(s) → Ca(OH)2(s) → (b) H3PO4(aq) + (c) NaOH(s) + H2SO4(aq) → (d) HNO3(aq) + NH4OH(aq) → (e) HCl(aq) + Pb(OH)2(s) → (f) Cu(OH)2(s) + HClO4(aq) → 2 5. Write complete, balanced equations for each of the following neutralization reactions. Use a solubility chart to help determine the phase of matter of the salt. (a) solid lithium hydroxide + phosphoric acid (b) hydroioidic acid + aqueous ammonium hydroxide (c) nitric acid + solid calcium hydroxide (c) suluric acid + solid aluminum hydroxide (d) hydrochloric acid + solid iron (III) hydroxide (e) sulfuric acid + aqueous barium hydroxide ACID / BASE TITRATION 6. Define the following words: (a) titration 3 6. Continued: (b) titrant (c) indicator (d) endpoint 7. (a) What is a commonly used indicator? (b) What color is this indicator in an acidic solution? (c) What color is this indicator in a basic solution? 8. A few drops of phenolphthalein are added to a 25.0-mL sample of an HCl solution. This solution is then titrated with a 0.185 M NaOH solution. (a) Which is the solution of unknown concentration? (b) What is the indicator? (c) What is the titrant? 4 8. Continued: (d) If 32.6 mL of the titrant were required to neutralize the HCl, what was the molarity of the HCl? 9. What is the molarity of an HCl solution if 28.6 mL of a 0.175 M NaOH solution is needed to neutralize a 25.0-mL sample of the HCl solution? 5 10. What volume, in milliliter, of a 0.115 M NaOH solution would neutralize 25.0 mL of a 0.106 M H2SO4 solution? 6 11. What volume, in milliliter, of a 0.158 M KOH solution would neutralize 50.0 mL of a 0.212 M HCl solution? 7