james chadwick, 1935
advertisement
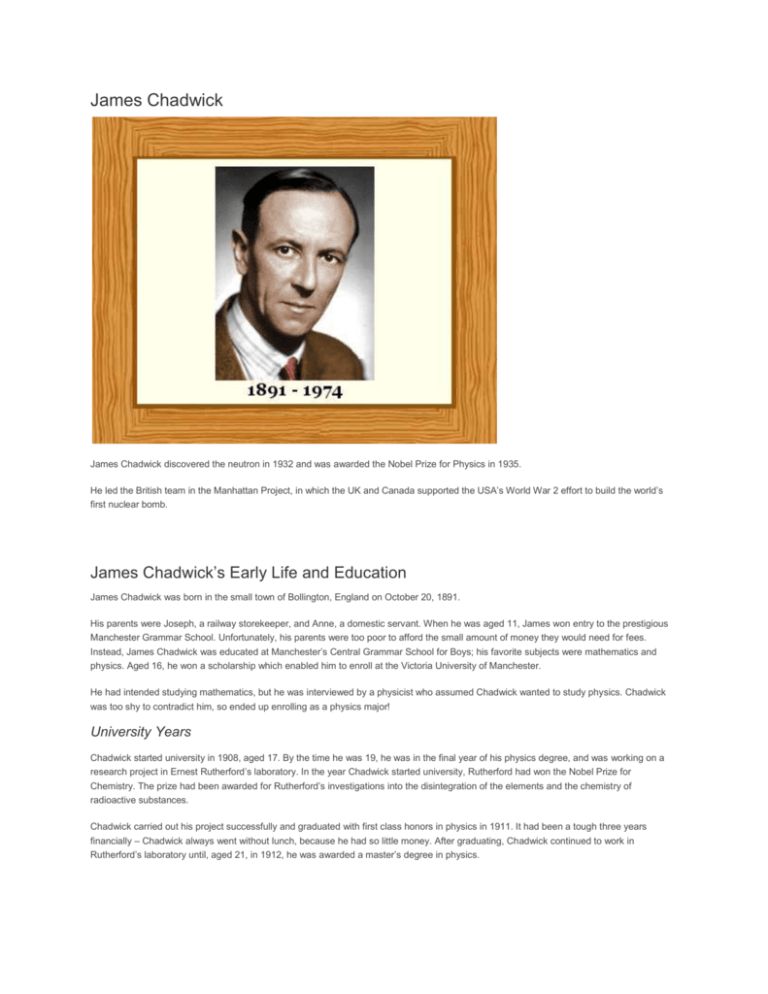
James Chadwick James Chadwick discovered the neutron in 1932 and was awarded the Nobel Prize for Physics in 1935. He led the British team in the Manhattan Project, in which the UK and Canada supported the USA’s World War 2 effort to build the world’s first nuclear bomb. James Chadwick’s Early Life and Education James Chadwick was born in the small town of Bollington, England on October 20, 1891. His parents were Joseph, a railway storekeeper, and Anne, a domestic servant. When he was aged 11, James won entry to the prestigious Manchester Grammar School. Unfortunately, his parents were too poor to afford the small amount of money they would need for fees. Instead, James Chadwick was educated at Manchester’s Central Grammar School for Boys; his favorite subjects were mathematics and physics. Aged 16, he won a scholarship which enabled him to enroll at the Victoria University of Manchester. He had intended studying mathematics, but he was interviewed by a physicist who assumed Chadwick wanted to study physics. Chadwick was too shy to contradict him, so ended up enrolling as a physics major! University Years Chadwick started university in 1908, aged 17. By the time he was 19, he was in the final year of his physics degree, and was working on a research project in Ernest Rutherford’s laboratory. In the year Chadwick started university, Rutherford had won the Nobel Prize for Chemistry. The prize had been awarded for Rutherford’s investigations into the disintegration of the elements and the chemistry of radioactive substances. Chadwick carried out his project successfully and graduated with first class honors in physics in 1911. It had been a tough three years financially – Chadwick always went without lunch, because he had so little money. After graduating, Chadwick continued to work in Rutherford’s laboratory until, aged 21, in 1912, he was awarded a master’s degree in physics. He then won a scholarship enabling him to go to Berlin, Germany to work in Hans Geiger’s laboratory. Like Rutherford, Geiger’s field was radioactivity, and Chadwick wished to continue his work in this field. Geiger’s name is remembered in the device he invented for detecting and measuring radiation – the Geiger Counter. Unfortunately for Chadwick, World War 1 began in 1914, when he was still in Berlin. He was interned in a camp on the west of Berlin until the war ended in 1918. Aged 28, in 1919, Chadwick rejoined Ernest Rutherford to begin working for his Ph.D. degree. Rutherford was now in charge of Cambridge University’s prestigious Cavendish Laboratory, whose first professor had been James Clerk Maxwell. Chadwick was awarded his Ph.D. in 1921 for a thesis concerning atomic numbers and nuclear forces. James Chadwick, the Nucleus and the Neutron Chadwick continued his nuclear research in the Cavendish Laboratory. In 1923, aged 32, he became Rutherford’s Assistant Director of Research, and continued to study the atomic nucleus. In those days, most researchers believed there were electrons within the nucleus as well as outside it. For example, the nucleus of an atom of carbon-12 was thought to contain 12 protons and 6 electrons, giving it an electric charge of +6. The 6 electrons orbiting the nucleus caused the overall electric charge on an atom of carbon to be 0. Rutherford, Chadwick, and some others believed in the possibility that particles with no charge could be in the nucleus. In his spare time, through the 1920s, Chadwick made a variety of attempts in the laboratory to find these neutral particles, but without success. He was, however, increasingly convinced in the existence of a neutral particle – the neutron. He couldn’t, however, get the evidence he needed to prove its existence. Then, at the beginning of 1932, Chadwick learned of work that Frederic and Irene Joliot-Curie had just done in Paris. The Joliot-Curies believed they had managed to eject protons from a sample of wax using gamma rays. This did not make sense to Chadwick, who thought gamma rays were not powerful enough to do this. However, the evidence that protons had been hit with sufficient energy to eject them was convincing. The gamma ray source had been the radioactive element polonium. Chadwick drew the conclusion that the protons had actually been hit by the particle he was looking for: the neutron. Feverishly, he began working in the Cavendish laboratory. Using polonium as a source of (what he believed were) neutrons, he bombarded wax. Protons were released by the wax and Chadwick made measurements of the protons’ behavior. The protons behaved in exactly the manner they ought to if they had been hit by electrically neutral particles with a mass similar to the proton. Chadwick had discovered the neutron. Within two weeks he had written to the prestigious science journal Nature to announce the Possible Existence of a Neutron. In fact, Chadwick at this time did not believe he had discovered a new elementary particle. He believed the neutron to be a complex particle consisting of a proton and an electron. The German physicist Werner Heisenberg showed that the neutron could not be an electron-proton pair, and was actually a new elementary particle. In 1935, James Chadwick received the Nobel Prize in Physics for his discovery of the neutron. An image from an expansion chamber in Chadwick’s laboratory. A neutron collides with an atom of nitrogen-14. The nitrogen atom splits into boron-11 and helium-4. And what of the Joliot-Curies? If only they had interpreted their results correctly, they might have discovered the neutron themselves. In fact, Frederic and Irene Joliot-Curie also received a Nobel Prize in 1935 – they took the prize in chemistry: they were the first people to create new, synthetic, radioactive elements. “I have already mentioned Rutherford’s suggestion that there might exist a neutral particle formed by the close combination of a proton and an electron, and it was at first natural to suppose that the neutron might be such a complex particle. On the other hand, a structure of this kind cannot be fitted into the scheme of the quantum mechanics,… the statistics and spins of the lighter elements can only be given a consistent description if we assume that the neutron is an elementary particle.” JAMES CHADWICK, 1935 Physicist New Elements and Nuclear Reactions The discovery of the neutron dramatically changed the course of science. It meant that neutrons could be collided with atomic nuclei. Some of the neutrons would imbed in a nucleus, increasing its mass. Natural Beta decay (the emission of an electron from an atom’s nucleus) would then convert the neutron into a proton. Since an element is defined by the number of protons it has (hydrogen has 1, helium 2, lithium 3, beryllium 4, boron 5, carbon 6, nitrogen 7, oxygen 8, etc, etc) this enabled scientists to make new, heavier elements in the laboratory. It also meant that neutrons could be used to split heavy atoms apart – atomic fission – producing a large amount of energy, which could be used in atomic bombs or nuclear power plants. Chadwick Leaves Cambridge for Liverpool In 1935, before his Nobel Prize was awarded, Chadwick was offered the Lyon Jones Chair of Physics at the University of Liverpool, which he accepted. He started his new job one month before he heard that he had won the Nobel Prize. In Liverpool, he started a nuclear physics group. The group needed a piece of equipment which could be described in a number of ways, including: cylclotron/particle-accelerator/atom smasher. His new university could not afford the cyclotron, so Chadwick part-funded it using some of his Nobel Prize money. World War 2 and the Atomic Bomb In 1939, the first year of World War 2, Chadwick was asked by the British Government about building an atomic bomb. He said, it was possible, but would not be at all easy. Preliminary research began in a number of universities. Working conditions were not easy in Chadwick’s laboratory in Liverpool. The neighborhood was frequently attacked in air-raids by the German Air Force. Despite the bombing, by spring 1941, Chadwick’s research group had discovered that the critical mass of uranium-235 for a nuclear detonation was about 8 kilograms. Wartime Liverpool after a German air-raid. Chadwick’s laboratory often shook from bomb blasts in the neighborhood. Chadwick wrote a summary report in summer 1941 of all the atomic bomb work carried out in British universities. In the USA, President Roosevelt read the report in the fall of 1941, and the USA started to pour millions of dollars into its own atomic bomb research. When he met them, Chadwick told American representatives that he was 90 percent sure the bomb would work. In late 1943, Chadwick traveled to the USA to see the Manhattan Project’s facilities. He was one of only three men in the world to enjoy access to all of America’s research, data, and production plants for the bomb: the other two were American Major General Leslie Groves, the Manhattan Project’s Director, and Groves’ second in command Major General Thomas Francis Farrell. Early in 1944, Chadwick, his wife, and children, moved to Los Alamos, the main research center for the Manhattan Project. Chadwick was present when the US and UK governments agreed that the bomb could be used against Japan. He then attended the Trinity nuclear test, on July 16, 1945, when the world’s first atomic bomb was detonated. In 1945, the British Government knighted him for his wartime contribution, and he became Sir James Chadwick. The U.S. Government awarded him the Medal of Merit in 1946, The End James Chadwick died peacefully, at the age of 82, on July 24, 1974.