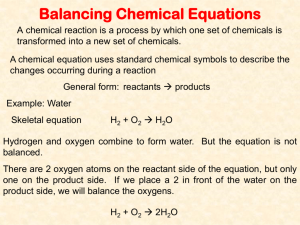
Balancing REDOX Equations Using The Half Reaction Method

Balancing REDOX Equations Using The Half-Reaction Method
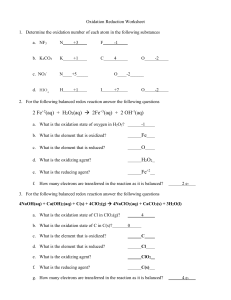
Writing the half reactions is surprisingly easy, if we stop to think about it. (I know, I know. It’s always easy after somebody shows it to you!) Let’s balance the overall REDOX equation for the manganate (VIII)/Fe 2+ titration.
1.
Given that aqueous manganate (VII) (permanganate) ions will be converted to Mn2+(aq) ion in acid solution, write a half equation to summarize this process.
MnO
4
is converted to Mn 2+ . So you might start this way:
MnO
4
Mn 2+ + 4O 2-
(not 2O
2
because Mn is metal & O isn’t, so ions)
But, that is 2+ on the left and 6- on the right, so we need 5 electrons on the left:
MnO
4
+ 5e
Mn 2+ + 4O 2-
But, how can we have a bunch of O 2 floating around in an acidic solution that is full of H + ? They will react to form water:
MnO
4
+ 5e
Mn 2+ + 4O 2 + 4H
2
O
But where did the H’s come from? Why, from the acid, H + :
MnO
4
+ 8H + + 5e
Mn 2+ + 4O 2 + 4H
2
O
And so we have the reduction of Mn from (VII) to (II). There is also an oxidation that must take place, so this is only half of the overall REDOX reaction, or the reduction half reaction.
2.
Write a half equation to summarize the conversion of Fe 2+ to Fe 3+ .
This much easier:
Fe 2+
Fe 3+ + e -
3.
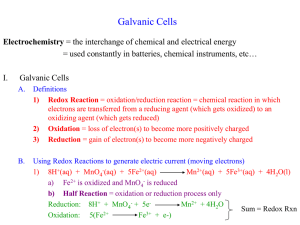
Combine the equations to form a complete REDOX equation, and clearly identify the oxidizing agent and the reducing agent.
So, combine them. But wait, that will give us 5 electrons on the left and 1 on the right. Where
did the extra electrons go? Simple….balance the electrons by multiplying the oxidation equation by 5 ( 5Fe 2+
5Fe 3+ + 5e ), and then combining the two half reactions.
MnO
4
+ 8H + + 5e + Fe 2+
Mn 2+ + 4O 2 + 4H
2
O + 5Fe 3+ + 5e -
Combining terms:
MnO
4
+ 8H + + 5Fe 2+
Mn 2+ + 4O 2 + 4H
2
O + 5Fe 3+
To summarize writing half reactions and balancing REDOX reactions:
1.
Identify what is reduced and what is oxidized by figuring out all the oxidation numbers and whether they gained or lost electrons;
2.
Write the ½ equation using only the oxidized or reduced species;
3.
Balance the non-oxygen and non-hydrogen elements;
4.
Add water molecules to the equation to balance oxygen;
5.
Add H + ions to balance hydrogen;
6.
Add e to balance the charge;
7.
Do the same for the other half reaction;
8.
Multiply both equations by appropriate integers to balance the electrons;
9.
Combine the two equations;
10.
Simplify the resulting complete REDOX equation.