Gas Laws, Intermolecular Forces and Solutions Review Session
advertisement

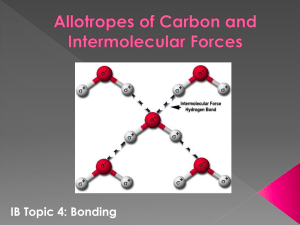
Name: ____________________________________________________ Gas Laws, Intermolecular Forces and Solutions Review Session: Gas Laws: Kinetic Molecular Theory (KMT): 1. 2. 3. 4. Gases have a very small volume Gases are constantly in moving at high speeds in random buts straight line paths Gases experience no electrostatic attractions between molecules The speed of a gas molecule is directly proportional to its mass and speed. These assumptions are good for IDEAL gases and are used quite often with little error. However, when one needs to be exact that you must account for the fact that REAL gases Have volume Experience electrostatic attractions Units of pressure Kilopascals = SI units of pressure, named after scientist Pascal Torr = named after scientist Torricelli mmHg = millimeters of mercury, comes from old way of measuring pressure by the height of a column of mercury open to atmosphere atm = atmosphere, unit most often used in chemistry. One atmosphere is what we feel experience every day. It is the standard pressure on earth. psi = pounds per square inch. Most often used in real life (tire pressure) Combined Gas Law Combining the above three laws allows us to calculate changes for systems in which volume, pressure, and temperature are changing. * temperature must be expressed in Kelvin * Notice that when 1. Temperature is constant, the equation becomes Boyle’s Law Example: heat is not added to the system and system is not insulated 2. Pressure is constant, the equation becomes Charles’ Law Example: system is open to air 3. Volume is constant, the equation becomes Gay-Lussac’s Law Example: system in enclosed in a non-expandable container such as a glass jar Example: What is the new volume of 10.0 L of gat at 1.05 atm and 100 K if the temp is increased to 255 K and the pressure is increased to 2.75 atm? o The kinetic molecular theory describes IDEAL gases o Most gases approximate ideal gases and the model works great for most gases. o Some REAL gases need adjustments o Real gases experience electrostatic attractions. o Real gases have volume Ideal gas law: PV = nRT o P = pressure measured in atm, kPa, mmHg, etc. o V = volume usually measured in L or dm3. o 1 L = 1 dm3 o n = number of moles of gas o R = ideal gas constant. Use the constant that best matches the pressure units given in the problem o R = 0.0821 L-atm/mol-K o R = 8.314 dm3-kPa/mol-K (SI unit – unit that will be on SOL) o R = 62.4 L-mmHg/mol-K o T = temperature, measured in Kelvin Example problems: 1. Calculate the volume of 10.0 moles of He gas at a pressure of 300. kPa and 50.0 C P= V= n= R= T= 2. Calculate the volume of 1.00 mol of H2 at STP STP = standard temperature pressure = P= V= n= R= T= Dalton’s Law of Partial Pressures Dalton’s Law states that: The total pressure of a mixture of gases is equal to the sum of the pressures of all the gases in the mixture. Ptotal = P1 + P2 + P3 + … The partial pressure of the gas depends on 1. the number of moles of gas, 2. The size of the container 3. the temperature of the mixture Example: A mixture of oxygen, carbon dioxide, and nitrogen has a total pressure 0.97 atm. What is the partial pressure of O2, if the partial pressure of CO2 is 0.70 atm and the partial pressure of N2 is 0.12 atm? Intermolecular Forces: How do atoms get 8 electrons? 1. The electrons are pulled towards the more electronegative atom giving it a partial negative charge - + O H = lower case Greek letter delta which means “partial” So Hydrogen has a partial positive charge (less than +1) This arrow gives the direction of the dipole moment for this bond. The positive pole is on the hydrogen and the negativity pole is on the oxygen atom (the negative pole is always on the more electronegative atom 2. Example: a. The two chlorine atoms each share one of their 2p valence electrons with the other so that they each have 8. b. This is shown more easily using Lewis Structures Cl Cl Chlorine c. d. Lewis structures show how the atoms are bonded and where all the electrons are located. How to determine the molecular polarity 1. Draw the Lewis structure 2. Identify polar bonds using the difference in electronegativity a. If there are no polar bonds AND no lone pairs the molecule is non-polar 3. Use VSEPR to figure out the shape of the molecule a. If the molecule is completely symmetrical the polar bonds may cancel each other out making the molecule non-polar F F C F Symmetrical molecule – non-polar even if the bonds are polar. F O H H Non-symmetrical molecule – polar if bonds are polar. b. If the molecule is not symmetric in every direction, than it is polar. i. Non-symmetric molecules have lone pairs on the central atom ii. The negative pole will be on the end of the molecule with the lone pairs or the end of the molecule with the most electronegative atom How to determine the intermolecular forces 1. Ionic – intermolecular forces is kind of a misnomer since there isn’t an ionic molecule but the forces that hold the formula units together are ionic bonds 2. Polar molecule (see above for finding molecular polarity) -intermolecular forces will be dipole-dipole forces and may be Hydrogen bonds if hydrogen is bonded to a very electronegative atom like Fluorine, Oxygen, or Nitrogen -polar molecules have permanent dipoles 3. Non-polar molecule -Intermolecular forces will be dispersion forces -Dispersion forces result from a temporary induced dipole -Random movement of electrons can result in a bunch of electrons located on one side of the molecule which gives that side of the molecule a slightly negative charge. A molecule near it will have a positive charge induced on it as the electrons in it move away from the negative charge. -The larger the molecule the more important dispersion forces become -Larger molecule means more electrons moving around so there is more chance for induced dipoles. How strong are intermolecular forces? 1. They are weaker than covalent and ionic bonds 2. Hydrogen bonds are usually stronger than generic dipole-dipole forces for molecules with the same mass Dipole-dipole forces are usually stronger than van der Waals forces for molecules with the same mass. As intermolecular forces increase, the molecules are held more strongly together. Solids resist melting because melting requires breaking intermolecular attractions and reforming new ones as the molecules slide past each other. Liquids resist boiling because the liquid molecules will have to overcome the intermolecular attraction of the other liquid molecules to enter the gas phase. Substance H2O NH3 HCl CH4 H2 Structural Diagram Boiling Point State at Room Temp IMF/ Relative Strength 100oC -33.3oC -85.1oC -164oC -252.9oC Phase Diagrams: A. Melting B. Freezing C. Vaporization D. Condensation E. Sublimation F. Deposition Solutions: 1. Dissociation When solutes dissolve the solute molecule is surrounded by water molecules If the solute is ionic, than the ions separate and are surrounded by water molecules NaCl Na+1 + Cl-1 + Na Cl+ Na Cl- + Na + Na Cl- Cl- + Na + Na + Cl- 2. Because of this, ionic substances have more of an effect on the boiling point and freezing point than covalent compounds -C6H12O6(s) C6H12O6(aq) -NaCl Na+1(aq) + Cl-1(aq) -CaCl2(s) Ca2+ (aq)+ 2Cl-1(aq) -Calcium chloride will have the largest effect and glucose, the least effect 3. Solutes that conduct electricity when dissolved are called “Electrolytes” Electrolytes are important for: Ca+2 movement allows muscles to contract and relax Presence of Na+ and K+ allow your nerve cells to respond to stimuli Weak electrolytes vs. Strong Electrolytes Strength of electrolyte depends on number of ions in solution More ions = strong electrolyte Fewer ions = weaker electrolyte 4. The rate at which a solution is formed is affected by: Surface area – Temperature- Agitation 5. Molarity (M)- the number of moles of solute dissolved in each liter of solution Calculate the molarity of a solution formed by mixing 10.0 g of sulfuric acid (H2SO4) with enough water to make 100.0 mL of solution. 6. Molality (m)- moles of solute per kilogram of solution (mol/kg) What is the molality of saltwater that contains 684 g of NaCl in 20.0 kg of water? SOL Review Questions: 1. A sample of nitrogen gas is collected over water at 20oC. The vapor pressure of water at 20oC is 18 mmHg. What is the partial pressure of the nitrogen if the total pressure is 765 mmHg? a. 747 mmHg b. 765 mmHg c. 783 mmHg d. 18 mmHg 2. One way to increase the volume of a balloon is toa. cool the gas in the balloon b. increase the temperature of the balloon c. push the balloon d. increase the pressure surrounding the balloon 3. A student needed to dissolve a substance that she knew was soluble in water. According to the charg, which other solvent would most likely dissolve the substance? a. benzene b. octane c. hexane d. methanol 4. One of the main assumptions of the kinetic molecular theory of gasses is that particles of an ideal gas a. must be single atoms instead of molecules b. are in constant motion c. must be highly chemically reactive d. must be maintained at high pressures Exit Ticket: You must answer all questions and fill out all information to receive the bonus points. 5/8/13 Review Session: Gas Laws, Intermolecular Forces and Solutions: Name: ______________________________________________ Teacher:_____________________ Block:________ 1. Write one question that you have regarding the material covered today. 2. A balloon has a volume of 15 L at 50oC at 0.750 atm, what is the volume at standard temperature and pressure? 3. What is the molarity of a solution that has 5 moles of sodium hydroxide in 2000 mL of solution? 4. Identify the strongest intermolecular force for the following compounds a. CH4 b. PF3 c. NH3