3 WHAT`S WRONG WITH COREY? Understanding what goes wrong
advertisement
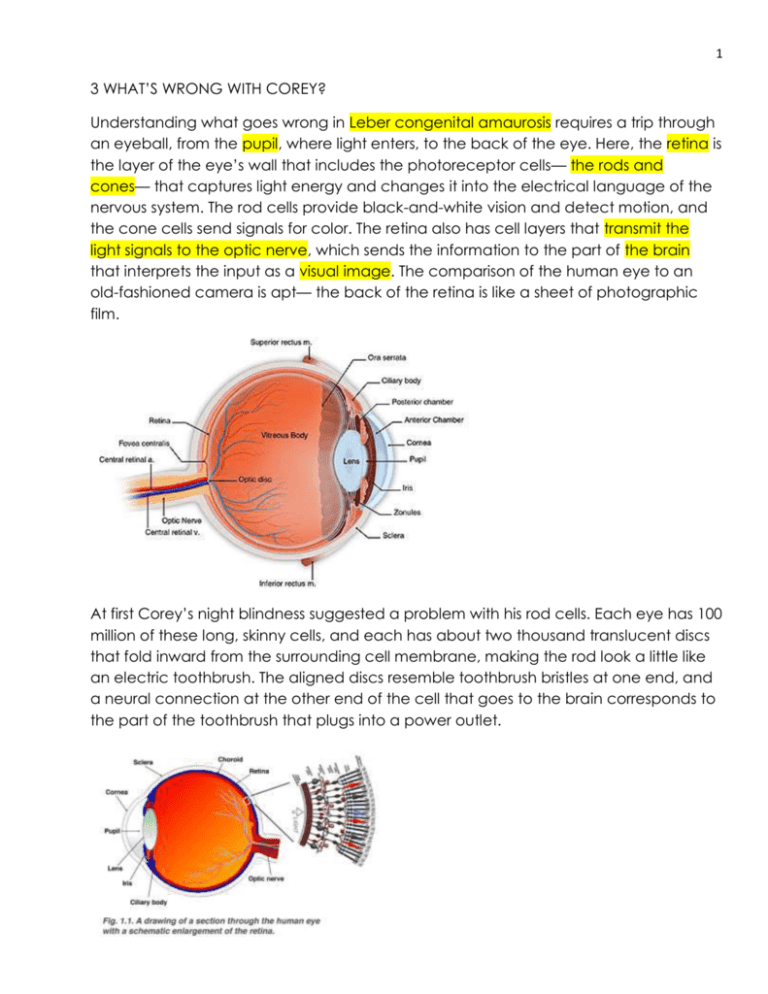
1 3 WHAT’S WRONG WITH COREY? Understanding what goes wrong in Leber congenital amaurosis requires a trip through an eyeball, from the pupil, where light enters, to the back of the eye. Here, the retina is the layer of the eye’s wall that includes the photoreceptor cells— the rods and cones— that captures light energy and changes it into the electrical language of the nervous system. The rod cells provide black-and-white vision and detect motion, and the cone cells send signals for color. The retina also has cell layers that transmit the light signals to the optic nerve, which sends the information to the part of the brain that interprets the input as a visual image. The comparison of the human eye to an old-fashioned camera is apt— the back of the retina is like a sheet of photographic film. At first Corey’s night blindness suggested a problem with his rod cells. Each eye has 100 million of these long, skinny cells, and each has about two thousand translucent discs that fold inward from the surrounding cell membrane, making the rod look a little like an electric toothbrush. The aligned discs resemble toothbrush bristles at one end, and a neural connection at the other end of the cell that goes to the brain corresponds to the part of the toothbrush that plugs into a power outlet. 2 Embedded in the rod’s folded discs are many molecules of a pigment called rhodopsin, which actually provides vision. Each rhodopsin molecule is built of a protein part called opsin and another, smaller part made from vitamin A, called retinal. A flash of light lasting mere trillionths of a second changes the shape of the retinal, which in turn changes the shape of the opsin. The change in opsin triggers chemical reactions that signal the nearby optic nerve, which stimulates the visual cortex in the brain. In this way, each of the 100 million rods and 3 million cones of a human eye contribute a tiny glimpse of a scene, which the brain then integrates into an image. To see the world as a continuous panorama, rather than a series of disconnected snapshots, rhodopsin must quickly reform after it changes in response to light. In dim light this happens slowly, and the rhodopsin is recycled inside the eye. But in very bright light, rhodopsin contorts too fast to fully recover. This is why we are temporarily blinded when walking out of a dark theater, to which our rhodopsin has adapted, into bright sunshine. It is also why we tell children to eat their carrots, because vitamin A deficiency causes night blindness. Cones work in a similar way, but instead of rhodopsin, they use three other visual pigments that are sensitive to different wavelengths of light, and interpret the hues of red, green, and blue that colors our world. Mammals other than humans and our primate cousins have only two types of cones, which restricts the color palette available to them. If Corey indeed had a form of Leber congenital amaurosis, the origin of his difficulty seeing wasn’t in his rods and cones, but in a layer of cells next to them called the retinal pigment epithelium. The RPE is a caretaker of sorts for the rods and cones, removing wastes while absorbing stray light rays that might otherwise bounce around the eyeball, creating meaningless flashes. The RPE’s most important job is to store vitamin A. It uses a protein, called RPE65, to activate the vitamin, forming the retinal essential for black-and-white vision. This would turn out to be Corey’s precise problem: his eyes can’t make RPE65. The condition is inherited, because genes tell cells how to make specific proteins. Without normal RPE65, nearly all of Corey’s rods and cones would shrivel away to nothing. By age forty— and that was being extremely 3 optimistic— he would be completely blind, and he would be legally and functionally blind far earlier. Yet because photoreceptors are so abundant that even the blindest of the blind still harbor a few of the cells, and the fact that Corey’s young age meant that many had not yet been starved, this particular form of LCA was an ideal candidate for gene therapy. And the younger the patient, the more likely it was to work. But LCA comes in at least eighteen different forms, each caused by a different abnormal gene. A genetic test would be necessary to turn Dr. Fulton’s notation in the medical record into a definitive diagnosis. * * * Its little wonder that it took years to identify the cause of Corey’s disappearing vision, since mutations in more than 180 genes can harm the retina. A common form of hereditary blindness is retinitis pigmentosa (RP), in which the photoreceptors themselves, especially the rods, shrink and then die. Symptoms of RP do not usually begin until early adulthood. Some researchers classify LCA as a form of RP, even though LCA is an indirect assault on the photoreceptors. However the classification works, the subtypes of LCA account for about 8 percent of inherited retinal dystrophies, but for 20 percent of children in schools for the blind, due to the early onset and severity. LCA was recognized long before researchers knew that it stems from mutations in any of eighteen-and-counting genes. A German ophthalmologist, Theodore Leber, first wrote about the familial form in 1869. However, early reports indicate that not all cases are congenital— some are environmental. A case report from 1886 described a young woman living in Savannah, Georgia, who developed the condition after taking too 4 much quinine to treat malaria. When she awoke from a coma, she couldn’t tell light from dark. The report is eerily like a description of Corey as a toddler attempting to navigate his living room. “She cannot see to go alone, but with care she can distinguish large colored objects in her room, and with some hesitation, constantly moving her eyes as she does so. She can count fingers at four feet, but she cannot make out a letter.” Luckily for her, the effects of quinine-induced amaurosis were temporary. Theodore Leber Amaurosis means “loss of sight.” One type of amaurosis, called “fugax,” stems from a fleeting circulatory disturbance in the eye, causing visual loss that lasts only a minute. It can be a warning sign of an impending stroke. Another form of amaurosis affects ruminants— cattle, sheep, goats, deer, and camels. They can develop the condition from vitamin B1 deficiency, which happens in two ways. One is when they eat certain ferns that disable a key enzyme. The second way is when there is a change in the bacterial populations in one of the four stomachs of such a creature, which prompts an explosive release of the foul anal gas hydrogen sulfide. * * * Corey returned to Boston Children’s Hospital on his fourth birthday. His condition was rapidly deteriorating, his tunnel of vision narrowing. Examining the backs of his eyes, Dr. Fulton found that each macula had developed a dark dimpled area in the center of the unusual pale circle. One bad eye could be a congenital fluke; two meant an inherited problem because the simultaneous occurrence of two rare events most likely stemmed from a fundamental error. Was it retinitis pigmentosa, perhaps the type that a mother who is a carrier passes to her son? But Nancy’s genetic test for it was normal. 5 Even as recently as 2004, genetic testing was painstakingly slow, as the analysis of the human genome sequence continued. Researchers had determined the entire DNA sequence in 2000, publishing final results by 2003. And so doctors would still try one or a few genetic tests at a time, those that seemed most appropriate as a child’s symptoms unfolded and simply because not many tests existed. Today not only are panels of genetic tests available for many diseases, but genome sequencing can identify mutations apparently unique to a particular family— this indeed happened for two LCA families who are friends of the Haases’ in the summer of 2011. But for Corey, genetic testing led in a logical, if slow, fashion toward his diagnosis. During the next visit to Boston, at the end of January 2005, it was Nancy and Ethan’s turn for ERGs, an experience that they do not remember with fondness because probes must be affixed directly to the eyeballs. Their scans showed low retinal function, but not low enough to affect their vision. This was a key clue. They could be carriers of the same form of inherited retinal disease, with symptoms so mild that they aren’t noticed. With these ERG results, the next step was to test the family’s DNA for LCA, because Corey’s flat ERG ruled out other inherited eye conditions. But even that wasn’t straightforward, because new genes were being discovered all the time. Ethan remembers this visit as a turning point. “Dr. Fulton asked us to reconsider the genetics, because a number of genes had been identified in research.” New genetic tests were becoming available. 6 Meanwhile, clinical clues also helped the doctors rule out certain other inherited eye diseases. They’d already eliminated conditions that might explain a child who sees better in the dark, the opposite of Corey (achromatopsia, cone dystrophy, or Stargardt disease), and concentrated on explanations for a child who sees better in the light (retinitis pigmentosa, congenital stationary night blindness, or some forms of LCA). Specialists had also crossed off the diagnosis list syndromes that include night blindness: Corey didn’t have the extra fingers or toes of Bardet-Biedl syndrome, the brain degeneration of Batten disease, the deafness of Usher syndrome, or the paleness of albinism. A general diagnosis of LCA is possible based on symptoms and test results such as the ERG, but genetic testing explains variations by identifying disease subtypes. Physicians and researchers use the different gene symbols, italicized abbreviations usually denoting the affected protein, in a language of sorts with which parents rapidly become quite fluent. Corey’s RPE65 form of LCA has mild improvement in early childhood, and then a course downward later in life, as do the CRB1 and LRAT subtypes. Children whose vision progressively but slowly declines could have AILP1 or RPERIP1, and infants born into total darkness could have the more common forms of LCA caused by mutation in the CEP290 gene or GUCY2D gene. Another diagnostic clue is that children with some types of LCA have a characteristic eye-poking behavior called the “oculodigital sign.” The appearance of the back of the eye holds clues, too. It is “blond” in some forms, like Corey’s, or may have a bull’s-eye-like center in another type. LCA comes in so many varieties because the functioning of the rods and cones is so complex that many proteins are involved in vision, and therefore many genes. Dr. Fulton’s careful medical record would help in selecting the specific genetic tests that would most likely finally answer the question “What’s wrong with Corey?” 7 • There are currently 7 well characterized forms of LCA: LCA(1): mutation in GUCY2D on 17p13.1; accounts for 21.2% of known LCA cases. LCA(?): mutation in CRB1 on 1q31; 10% of known LCA cases. LCA(2): mutation in RPE65 on 1p31; 6.1% of known LCA cases. LCA(6): mutation in RPGRIP on 14q11; 4.5% of known LCA cases. LCA(4): mutation in AIPL1 on 17p13.1; 3.4% of known LCA cases. LCA(?): mutation in TULP1 on 6q21.3; 1.7% of known LCA cases. LCA(?):mutation in CRX on 19q13.3; 0.6% of known LCA cases Dr. Harris, the geneticist at Boston Children’s Hospital, sent blood from the Haas family to the John and Marcia Carver Nonprofit Genetic Testing Laboratory. Unlike other facilities whose offerings cover many body parts, the Carver lab specializes in the eye. They test for most, but not all, of the genes known to cause LCA. “Looking for LCA genes in the human genome is not like looking for a needle in a haystack, but like looking for a silver needle in a haystack of steel needles,” explained Edwin Stone, MD, PhD, director of the lab and professor of ophthalmology and visual sciences at the University of Iowa in nearby Iowa City. He was speaking at a meeting for LCA families on the University of Pennsylvania campus on a midsummer weekend in 2010. The lab is not-for-profit, but testing usually isn’t free because sequencing entire genes, which may be necessary to spot a rare mutation, is expensive. Finding a common LCA mutation might cost only $ 700, but if it’s rare, the charge goes up by $ 600. The price tag for an accelerated, six-week turnaround is $ 2,500. About a quarter of families who submit blood samples to the Carver lab don’t get an answer, and end up paying the fee to eliminate possibilities. They then seek out other facilities that test for additional mutations, such as the DNA Diagnostic Lab at the University of Colorado in Denver. If answers keep coming back negative, families have their entire “exomes” (the part of the genome that encodes protein) sequenced. A decade from now, once researchers have identified all human genes and their variants, finding a mutation that causes a specific disease will be as easy as typing a book title into Amazon.com. 8 To speed genetic-testing identification of the estimated three thousand people who have LCA, the Carver lab is running Project 3000. Wyc Grousbeck, owner of the Boston Celtics, and Derrek Lee, first baseman of the Chicago Cubs, started the project in 2005, after each had a child diagnosed with the disorder. (Derrek Lee’s daughter turned out to have a viral infection and not LCA, but he’s stayed involved.) If a family lacks health insurance to cover testing, donations may defray costs. I was initially put off by denials of my requests to visit the Carver lab or even to speak to Dr. Stone, but then I learned that he works nearly round-the-clock, tracking down genes, developing new tests, and talking to frantic parents at all hours. I found myself next to him at the LCA family meeting, and tried to explain that I needed to talk to him for this book. Just then, the speaker announced that Dr. Stone would be available to meet with parents for ten-minute time slots and pointed him out, and he was instantly mobbed. He smiled at me as parents lined up, and he shrugged. “See the problem?” Fortunately, Corey’s LCA mutation is so common that eight labs test for it, including Carver. The family finally traveled to Boston on July 5, 2006, to learn the results from Dr. Fulton. Dr. Edwin Stone 9 * * * Corey was now nearing his sixth birthday. He was making excellent progress in learning Braille, but he needed so much paraphernalia in his classroom in order to see that he and his stuff took up two seats. The teacher put him in the back of the room because his equipment distracted the other kids if he sat in the front. He remembers the isolation well. “I had a Clarity DeskMate, which is like a little computer monitor screen with an arm with a camera attached that let me see the whiteboard. I had to sit in the back.” To read at his seat, Corey needed a magnifying glass and a 100-watt bulb, and still he had to keep his face right next to the print. Clarity DeskMate “If Corey was in a typical classroom, where the kids would find the light bright enough to read, he could not. His pupils would dilate as far as they could, as if they were trying to pull every photon out of the room,” explained Dr. Jean. Dr. Fulton told Nancy and Ethan that Corey had inherited a different mutation, but in the same gene, from each of them. This meant they weren’t distant cousins, which is always a possibility with an extremely rare genetic disease. Relatives can inherit the same mutation from a shared ancestor, such as a common great-grandparent. Ethan’s genetic quirk is localized: one DNA base subs for another, like a one-letter typo. Nancy’s class of mutation, called “nonsense,” prematurely halts her cells’ reading of the gene, releasing just a nubbin of RPE protein, if any at all. (“ I always thought you were full of nonsense!” joked Ethan.) As carriers, Nancy and Ethan could see because each has a working copy of the gene. Team their two mutations in the eyes of a child, however, and his cells can’t make the needed RPE65 protein at all. Corey’s rods were starving— and soon his cones would be, too. 10 The RPE65 gene is located at 1p31.1 and is about 21,000 base pairs long. Ethan and Nancy couldn’t recall much of the Genetics 101 discussion they had with Dr. Fulton in Boston on that July day in 2006. Despite the years of doctor visits, and the mounting clues that Corey had inherited something, they were still in shock. But they managed to hear enough to realize that each child of theirs stood a 25 percent chance of having Corey’s disease. One single word stopped them from absorbing further genetic details. “I remember when I first heard the word blind. I thought immediately of all Corey wouldn’t be able to do,” says Nancy years later, still tearing up at the memory. Dr. Fulton’s recommendations were bleak. Corey should continue his vision-support services at school and wear his glasses, she said kindly, and continue his efforts to learn Braille. He needn’t return to Boston for another year, because there was nothing more they could do. She did, however, mention ongoing research, but that flew right past the distraught parents trying to comprehend that their child, their only child, was going blind from something that they had given him. The medical report for that visit ends with the prophetic words, “It is the RPE65 type of Leber congenital amaurosis that recently appears to have responded to some experimental treatment. This is a hopeful beginning.” Indeed it was— but Corey’s terrified parents didn’t yet know it. What they did know, finally, was the enemy: a specific mutation in a specific gene. Corey hadn’t overdosed on malaria meds, or suffered a stroke, nor was he a flatulent camel. He was, however, a zebra. Fledgling medical students learn right away the mantra “When you hear hoof beats, think horses, not zebras.” It means suspect first the most common explanation for a patient’s symptoms. Round up the usual suspects. In Corey’s case the horses were night blindness and albinism, then retinitis pigmentosa. Medical geneticists, however, deal almost exclusively with zebras, most of which are caused by mutations in single genes. Even the more familiar among these, such as cystic fibrosis and sickle cell disease, are rare compared with conditions that reflect more of an environmental input, such as the common forms of heart disease or emphysema. But understanding how the rare genetic conditions happen can often explain more common ills. For 11 example, the statin drugs that millions of people take to lower cholesterol were developed based on studying the one-in-a-million children who died of familial hypercholesterolemia, their cholesterol so high that it collected in waxy yellow clumps behind their knees and elbows. Understanding how the mutant gene revved up the body’s own cholesterol production suggested ways to induce the opposite effect. Familial hypercholesterolemia From finally putting a name to their son’s blindness until the next appointment in Boston a year later, Ethan and Nancy navigated a personal hell of helplessness and despair as Corey’s visual world continued to close in on him. They also struggled with the special guilt that comes with an inherited illness. “You think, how on earth could this happen to your son? How did I cause this? Then, how am I going to fix it?” recalls Ethan, his and Nancy’s anguish easily reawakened. They’d decided not to risk having other children, and were steeling themselves for raising a blind child. A year later, at the appointment at Boston Children’s Hospital on June 28, 2007, everything changed. Dr. Fulton wrote in the record, “A new experimental treatment of gene replacement is planned. We are told that the youngest patients will be considered for this treatment is 8 years, an age that Corey is approaching.” She told Nancy and Ethan to bring Corey back in a year, and then she called Dr. Jean Bennett, at CHOP. But Corey never returned to Boston. Instead, he had gene therapy. Despite Corey’s misfortune in the game of genetic roulette, he was very lucky to be in the right place at the right time. His diagnosis, although it took years of detours, came just as researchers were about to launch a clinical trial to test a gene therapy for not only his rare type of visual loss, but for his subtype. The goal: give Corey working copies of the errant gene. Like most people, Ethan and Nancy Haas had never heard of gene therapy. They had no way to know that Dr. Fulton’s note in the medical record would lead to Corey’s participating in an experiment that would land him on radio and TV and in headlines around the world, even to being named a “breakthrough of the year” in Science magazine. They couldn’t know that one day Dr. Francis Collins, the director of the National Institutes of Health, would show the U.S. Congress a video of Corey navigating an obstacle course, stumbling with his treated eye patched, then zipping 12 through in seconds with his untreated eye patched. Dr. Collins, grinning before Congress, shouted, “Go, Corey, go!” as the audience watched the video. Corey’s newfound vision has had a galvanizing effect on the field of gene therapy for several reasons. His plight was easy to relate to— everyone can imagine what it must be like to be so young in a darkening world. A bright, charming child with rosy cheeks and a wide grin, Corey has never fit the image of a person with a genetic disease. And the gene therapy clearly worked. But to understand how Corey figuratively, and perhaps literally, saved gene therapy, his success must be considered against the backdrop of a biotechnology derailed, seemingly doomed, by failure and tragedy. Earlier gene therapy attempts, also on children but for different and more lifethreatening conditions, led to questionable or only incremental or transient improvements. For certain inherited brain diseases, gene therapy only slowed the deterioration, extending life but leaving teens with lingering and profound disabilities. And then, in 1999, in another late September in Philadelphia, involving one of the same researchers, an eighteen-year-old had gene therapy for a liver disease. Jesse Gelsinger was the first, but not the last, to die from gene therapy. Jesse Gelsinger