SOLUTION_ASSIGNMENT_CHAPTER 3
advertisement
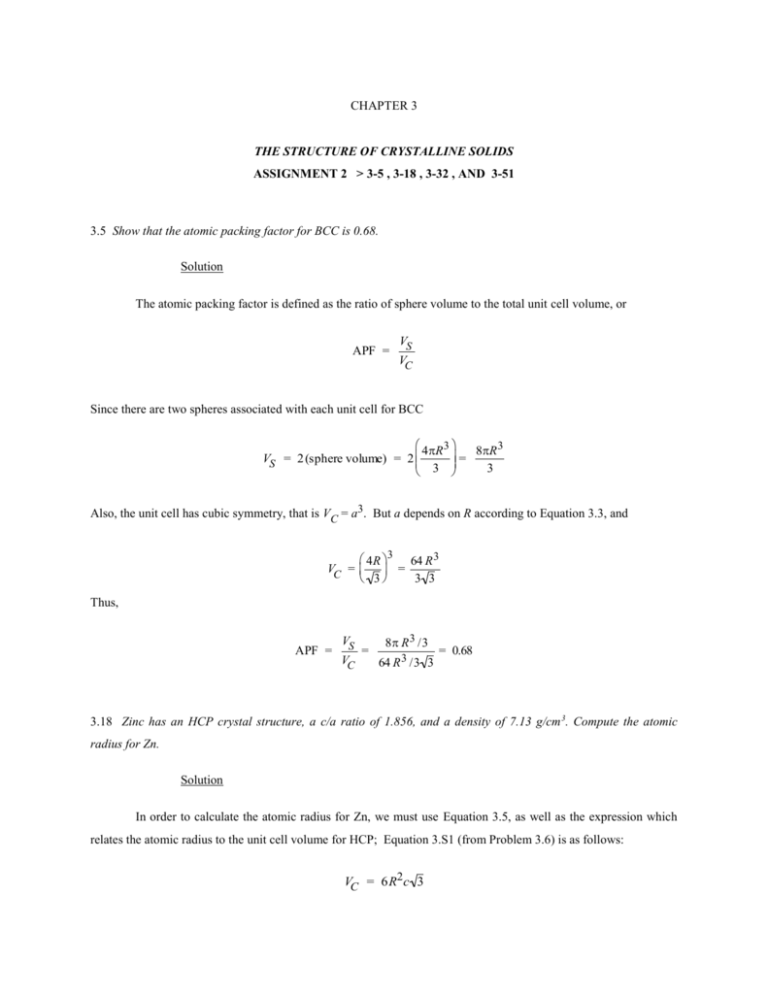
CHAPTER 3 THE STRUCTURE OF CRYSTALLINE SOLIDS ASSIGNMENT 2 > 3-5 , 3-18 , 3-32 , AND 3-51 3.5 Show that the atomic packing factor for BCC is 0.68. Solution The atomic packing factor is defined as the ratio of sphere volume to the total unit cell volume, or APF = VS VC Since there are two spheres associated with each unit cell for BCC 4R3 8R3 VS = 2 (sphere volume) = 2 3 = 3 Also, the unit cell has cubic symmetry, that is VC = a3. But a depends on R according to Equation 3.3, and 3 4R 64 R 3 VC = = 3 3 3 Thus, APF = VS VC = 8 R 3 /3 64 R 3 /3 3 = 0.68 structure, a c/a ratio of 1.856, and a density of 7.13 g/cm 3. Compute the atomic 3.18 Zinc has an HCP crystal radius for Zn. Solution In order to calculate the atomic radius for Zn, we must use Equation 3.5, as well as the expression which relates the atomic radius to the unit cell volume for HCP; Equation 3.S1 (from Problem 3.6) is as follows: VC = 6R2c 3 In this case c = 1.856a, but, for HCP, a = 2R, which means that VC = 6 R2 (1.856)(2R) 3 (1.856)(12 3)R3 And from Equation 3.5, the density is equal to = nAZn nAZn = VC N A (1.856)(12 3)R3 N A And, solving for R from the above equation leads to the following: 1/3 nAZn R = (1.856) (12 3) N A And incorporating appropriate values for the parameters in this equation leads to 1/3 (6 atoms/unit cell) (65.41 g/mol) R = 3 23 (1.856) (12 3)(7.13 g/cm )(6.022 10 atoms/mol ) = 1.33 10-8 cm = 0.133 nm 3.32 Determine the indices for the directions shown in the following cubic unit cell: Solution Direction A is a [430] direction, which determination is summarized as follows. We first of all position the origin of the coordinate system at the tail of the direction vector; then in terms of this new coordinate system x y 2a b 3 2 2 1 3 2 Projections – Projections in terms of a, b, and c – Reduction to integers Enclosure –4 z 0c 0 3 0 [430] Direction B is a [ 23 2] direction, which determination is summarized as follows. We first of all position the origin of the coordinate system at the tail of the direction vector; then in terms of this new coordinate system Projections x y 2a –b 3 2 Projections in terms of a, b, and c Enclosure 2c 3 2 –1 3 Reduction to integers z –3 2 [ 23 2] 3 2 Direction C is a [13 3] direction, which determination is summarized as follows. We first of all position the origin of the coordinate system at the tail of the direction vector; then in terms of this new coordinate system Projections x y z a –b –c –1 –1 –3 –3 3 1 Projections in terms of a, b, and c 3 Reduction to integers Enclosure 1 [13 3] Direction D is a [136 ] direction, which determination is summarized as follows. We first of all position the origin of the coordinate system at the tail of the direction vector; then in terms of this new coordinate system Projections Projections in terms of a, b, and c Reduction to integers x y z a b 6 1 2 1 –c 6 2 1 3 –1 –6 [136 ] Enclosure 3.55 (a) Derive planar density expressions for BCC (100) and (110) planes in terms of the atomic radius R. (b) Compute and compare planar density values for these same two planes for vanadium. Solution (a) A BCC unit cell within which is drawn a (100) plane is shown below. For this (100) plane there is one atom at each of the four cube corners, each of which is shared with four adjacent unit cells. Thus, there is the equivalence of 1 atom associated with this BCC (100) plane. The planar section 4R represented in the above figure is a square, wherein the side lengths are equal to the unit cell edge length, 3 4R 2 16 R 2 (Equation 3.3); and, thus, the area of this square is just 3 = 3 . Hence, the planar density for this (100) plane is just PD100 = number of atoms centered on (100) plane area of (100) plane 1 atom 16 R 2 3 16 R 2 3 A BCC unit cell within which is drawn a (110) plane is shown below. For this (110) plane there is one atom at each of the four cube corners through which it passes, each of which is shared with four adjacent unit cells, while the center atom lies entirely within the unit cell. Thus, there is the equivalence of 2 atoms associated with this BCC (110) plane. The planar section represented in the above figure is a rectangle, as noted in the figure below. From this figure, the area of the rectangle is the product of x and y. The length x is just the unit cell edge length, 4R which for BCC (Equation 3.3) is . Now, the diagonal length z is equal to 4R. For the triangle bounded by the 3 lengths x, y, and z y z 2 x2 Or y 4R 2 4R 2 (4 R) 2 3 3 Thus, in terms of R, the area of this (110) plane is just 4 R 4 R 2 16 R2 2 Area (110) xy 3 3 3 And, finally, the planar density for this (110) plane is just PD110 = number of atoms centered on (110) plane area of (110) plane 2 atoms 16 R2 2 3 8 R2 2 3 (b) From the table inside the front cover, the atomic radius for vanadium is 0.132 nm. Therefore, the planar density for the (100) plane is PD 100(V) 3 3 10.76 nm2 1.076 10 19 m2 2 16 R 16 (0.132 nm) 2 While for the (110) plane PD 110(V) 3 3 15.22 nm2 1.522 10 19 m2 8 R 2 2 8 (0.132 nm) 2 2 3.51 Sketch the (11 01) and (112 0) planes in a hexagonal unit cell. Solution For (1 1 01) the reciprocals of h, k, i, and l are, respectively, 1, –1, , and 1; thus, this plane is parallel to the a3 axis, and intersects the a1 axis at a, the a2 axis at –a, and the z-axis at c. The plane having these intersections is shown in the figure below For (112 0) the reciprocals of h, k, i, and l are, respectively, 1, 1, –1/2, and ; thus, this plane is parallel to the z axis, and intersects the a1 axis at a, the a2 axis at a, and the a3 axis at –a/2. The plane having these intersections is shown in the figure below. PD 110(V) 3 3 15.22 nm2 1.522 10 19 m2 8 R 2 2 8 (0.132 nm) 2 2