Quiz #2
advertisement

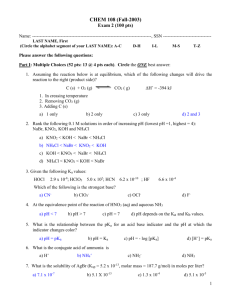
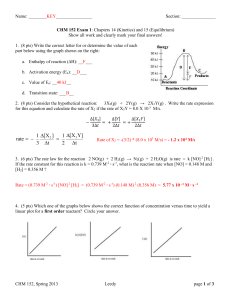
Quiz #2 – CHEM 220 Spring 2013 1. (6 pts TOTAL) Answer questions a-d according to the following equilibrium: HF (aq) H+ (aq) + F- (aq) ; ∆H = - 74.85 KJ/mol ; ∆S = +130.4 J/K mol; at 25 °C a. (3 pts) If you increase the temperature of this reaction to 227 °C, will the equilibrium shift to increase reactants or to increase products? Explain. b. (3 pts) Do you predict K(equilibrium constant) to be greater or less than 1? Explain. 2. (8 pts) Calculate the value of K for the reaction: 2 N2O(g) + 3 O2(g) the following information. Think of the gases as aqueous solutions. Equation 2 N2 (g) + O2(g) N2O4(g) 2 N2O(g) 2 NO2(g) ½ N2(g) + O2(g) NO2(g) 2 N2O4(g), using Equilibrium Constant K = 1.2 x 10-35 K = 4.6 x 10-3 K = 4.1 x 10-9 3. (6 pts) Is it possible to separate 0.100M Mg+2 from 0.100M Ca+2 with a 85% separation? Use the information below that you think you need. For full credit, you need absolute proof via calculations and a complete explanation of your experiment. Ksp MgCO3(s) = 3.5 x 10-8 Ksp MgF2(s) = 7.4 x 10-9 Ksp CaCO3(s) = 6.0 x 10-9 Ksp CaF2(s) = 3.2 x 10-11 4. (15 pts TOTAL) a. (4 pts) What is the equilibrium[Cu2+] of a saturated solution of Cu(OH)2 (s) ? Ksp Cu(OH)2 (s) = 4.8 x 10-20 b. (6 pts) What is the equilibrium [Cu2+] of this same solution if there is also 0.01 M Mg(OH)2 present? Ksp Cu(OH)2 (s) = 4.8 x 10-20 c. (3 pts) Would you predict there to be a difference in the equilibrium [Cu2+] if there was 0.01 M Ca(NO3)2 present instead of 0.01 M Mg(OH)2 present? Explain your pick qualitatively and thoroughly. d. (1 pt) Name Ca(NO3)2 e. (1 pt) Name Mg(OH)2 BONUS (+ 2 pts) 1. If the pH of a solution is 4, what is the [H+] concentration? 2. If the [OH-] of a solution is 1 x 10-4M, what is the pH of that solution?