Examples of Active Grants Since 2006
advertisement
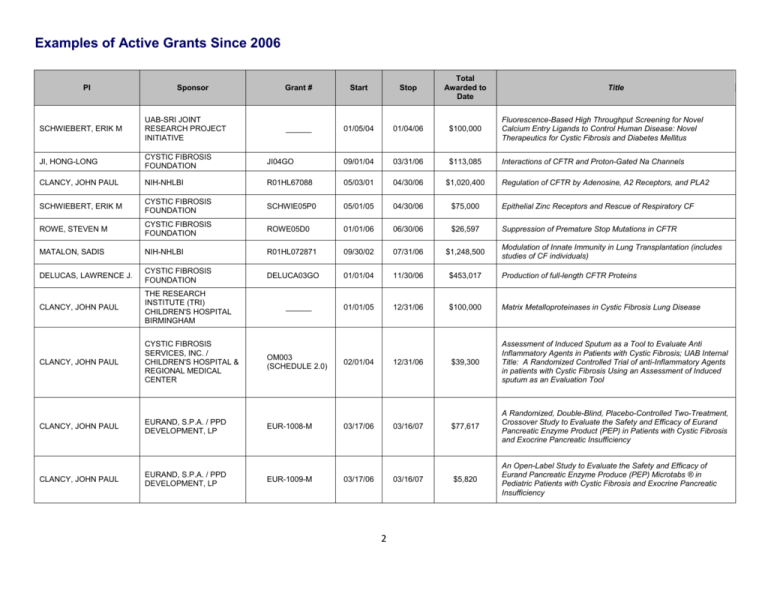
Examples of Active Grants Since 2006 PI Sponsor Grant # Start Stop Total Awarded to Date ______ 01/05/04 01/04/06 $100,000 Fluorescence-Based High Throughput Screening for Novel Calcium Entry Ligands to Control Human Disease: Novel Therapeutics for Cystic Fibrosis and Diabetes Mellitus Interactions of CFTR and Proton-Gated Na Channels Title SCHWIEBERT, ERIK M UAB-SRI JOINT RESEARCH PROJECT INITIATIVE JI, HONG-LONG CYSTIC FIBROSIS FOUNDATION JI04GO 09/01/04 03/31/06 $113,085 CLANCY, JOHN PAUL NIH-NHLBI R01HL67088 05/03/01 04/30/06 $1,020,400 SCHWIEBERT, ERIK M CYSTIC FIBROSIS FOUNDATION SCHWIE05P0 05/01/05 04/30/06 $75,000 Epithelial Zinc Receptors and Rescue of Respiratory CF ROWE, STEVEN M CYSTIC FIBROSIS FOUNDATION ROWE05D0 01/01/06 06/30/06 $26,597 Suppression of Premature Stop Mutations in CFTR MATALON, SADIS NIH-NHLBI R01HL072871 09/30/02 07/31/06 $1,248,500 DELUCAS, LAWRENCE J. CYSTIC FIBROSIS FOUNDATION DELUCA03GO 01/01/04 11/30/06 $453,017 Production of full-length CFTR Proteins CLANCY, JOHN PAUL THE RESEARCH INSTITUTE (TRI) CHILDREN'S HOSPITAL BIRMINGHAM 01/01/05 12/31/06 $100,000 Matrix Metalloproteinases in Cystic Fibrosis Lung Disease CLANCY, JOHN PAUL CYSTIC FIBROSIS SERVICES, INC. / CHILDREN'S HOSPITAL & REGIONAL MEDICAL CENTER OM003 (SCHEDULE 2.0) 02/01/04 12/31/06 $39,300 Assessment of Induced Sputum as a Tool to Evaluate Anti Inflammatory Agents in Patients with Cystic Fibrosis; UAB Internal Title: A Randomized Controlled Trial of anti-Inflammatory Agents in patients with Cystic Fibrosis Using an Assessment of Induced sputum as an Evaluation Tool CLANCY, JOHN PAUL EURAND, S.P.A. / PPD DEVELOPMENT, LP EUR-1008-M 03/17/06 03/16/07 $77,617 A Randomized, Double-Blind, Placebo-Controlled Two-Treatment, Crossover Study to Evaluate the Safety and Efficacy of Eurand Pancreatic Enzyme Product (PEP) in Patients with Cystic Fibrosis and Exocrine Pancreatic Insufficiency CLANCY, JOHN PAUL EURAND, S.P.A. / PPD DEVELOPMENT, LP $5,820 An Open-Label Study to Evaluate the Safety and Efficacy of Eurand Pancreatic Enzyme Produce (PEP) Microtabs ® in Pediatric Patients with Cystic Fibrosis and Exocrine Pancreatic Insufficiency ______ EUR-1009-M 03/17/06 03/16/07 2 Regulation of CFTR by Adenosine, A2 Receptors, and PLA2 Modulation of Innate Immunity in Lung Transplantation (includes studies of CF individuals) Examples of Active Grants Since 2006 PI Sponsor Grant # Start Stop Total Awarded to Date ______ 01/01/05 03/31/07 $189,605 Matrix Metalloproteinases: Mediators of Acute Lung Injury in Children Title CLANCY, JOHN PAUL THRASHER RESEARCH FUND CLANCY, JOHN PAUL SALUS PHARMA / CORUS PHARMA CP-AI-0007 04/25/06 04/24/07 $37,328 A Phase 3, Double-Blind, Multicenter, Multinational, Randomized, Placebo-Controlled Trial Evaluating Aztreoman Lysinate for Inhalation in Cystic Fibrosis Patients with Pulmonary P. aeruginosa (AIR-CFI) BEBOK, ZSUZSANNA PTC THERAPEUTICS, INC. PTC124-GD-003CF 01/30/06 04/30/07 $38,973 Morphologic studies following administration of new PTC compounds as oral treatment for nonsense-mediated cystic fibrosis BEDWELL, DAVID M NIH-NIGMS R01GM68854 06/01/03 05/31/07 $1,137,207 FU, LIANWU (KARL) / SZTUL, ELIZABETH NIH-NIDDK K01 068074 07/01/04 06/30/07 $288,036 Sorting of CFTR for Degradation CLANCY, JOHN PAUL INSPIRE PHARMACEUTICALS, INC 08/07/06 08/06/07 $59,991 A Multicenter, Double-Blind, Placebo-controlled Randomized, Efficacy and Safety study of Denufosol Tetrasodium (INS37217) Inhalation Solution in Patients with Mild Cystic Fibrosis Lung Disease BENOS, DALE J NIH-NIDDK P50DK53090 09/01/02 08/31/07 $1,080,913 BEDWELL, DAVID M NIH-NIDDK P50DK53090 09/01/03 08/31/07 $553,906 Mechanistic Studies of CF Pathogenesis and CI Secretion - CF Mouse Core CLANCY, JOHN PAUL CYSTIC FIBROSIS FOUNDATION ______ 09/01/06 08/31/07 $20,185 Examination of the metabolome of CFBE cell monolayers stably transduced with lentivral cDNAs coding for wt CFTR, and DF508CFTR compared with parental cells expressing endogenous DF508 CFTR KIRK, KEVIN L NIH-NIDDK P50DK53090 09/01/02 08/31/07 $1,080,913 SCHWIEBERT, ERIK M NIH-NIDDK P50DK53090 09/01/02 08/31/07 $218,633 Mechanistic Studies of CF Pathogenesis and CI Secretion - Core B SORSCHER, ERIC J NIH-NIDDK P50DK53090 09/01/02 08/31/07 $216,493 Mechanistic Studies of CF Pathogenesis and CI Secretion - Core C BEDWELL, DAVID M NIH-NIDDK P50DK53090 09/01/02 08/31/07 $1,080,913 3 Mechanism of Eukaryotic Translation Termination Mechanistic Studies of CF Pathogenesis and CI Secretion Project 2 Mechanistic Studies of CF Pathogenesis and CI Secretion Project 1 Mechanistic Studies of CF Pathogenesis and CI Secretion Project 3 Examples of Active Grants Since 2006 Sponsor Grant # Start Stop Total Awarded to Date CLANCY, JOHN PAUL PTC THERAPEUTICS / CHILDREN'S HOSPITAL AND REGIONAL MEDICAL CENTER PTC124-GD-003CF 09/21/05 09/30/07 $215,050 A Phase 2 Study of PTC124 as an Oral Treatment for NonsenseMutation-Mediated Cystic Fibrosis GUTIERREZ, HECTOR H ALABAMA DEPARTMENT OF PUBLIC HEALTH C70119154 05/01/07 09/30/07 $23,285 Cystic Fibrosis Screening for Newborn Infants CLANCY, JOHN PAUL CYSTIC FIBROSIS FOUNDATION / UNIVERSITY OF NORTH CAROLINA CHAPEL HILL 223265 05/07/04 12/01/07 $3,800 Genetic Modifiers in Cystic Fibrosis Lung Disease GUTIERREZ, HECTOR H GENENTECH, INC. Z3877G 04/01/07 03/31/08 $28,462 A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of Pulmozyme ® Withdrawal on Exercise Tolerance in Cystic Fibrosis Patients ROWE, STEVEN M NOVARTIS PHARMACEUTICALS CORPORATION A two-part, randomized, double-blind, placebo-controlled, ascending single-dose, adaptive study to evaluate safety, tolerability, pharmacokinetics and pharmacodynamics of QAU145 administered via a nasal spray pump to patients with cystic fibrosis PI CLANCY, JOHN PAUL BROUILLETTE, CHRISTIE CHILDREN'S HOSPITAL & REGIONAL MEDICAL CENTER UAB RESEARCH FOUNDATION Title QAU145A2201 06/11/07 04/08/08 $174,693 412380260101UAB 07/01/07 06/30/08 $2,430 Antimicrobial resistance in sputum obtained from patients with cystic fibrosis (CF) ______ 08/02/06 09/23/08 $33,469 Testing of SGX/CFFT Proteins and Ligands ______ 09/01/06 09/30/08 $120,000 Estrogen Sulfation in the Dysregulation of Hepatocyte Growth Hormone Signaling in Cystic Fibrosis 10/01/07 09/30/08 $68,379 Cystic Fibrosis Screening for Newborn Infants ______ 10/01/07 09/30/08 $20,313 Detection of CFTR in Airway Cells from CF Patients Regulation of Epithelial Sodium channels by cGMP FALANY, CHARLES NAPOLEON CYSTIC FIBROSIS RESEARCH, INC. GUTIERREZ, HECTOR H ALABAMA DEPARTMENT OF PUBLIC HEALTH CLANCY, JOHN PAUL VERTEX PHARMACEUTICALS, INC. JI, HONG-LONG / MATALON, SADIS NIH-NHLBI R01HL87017 01/01/07 01/31/09 $171,684 ROWE, STEVEN M CYSTIC FIBROSIS FOUNDATION ROWE08AO 02/01/08 01/31/09 $2,700 Examination of the Metabolome of CF and non-CF Tissues from Nasal Polyps CLANCY, JOHN PAUL GILEAD SCIENCES, INC. CP-AI-0006 02/01/07 01/31/09 $45,503 A Phase 3, Open-Label, Follow-on Study of Multiple Courses of Aztreonam Lysinate for Inhalation (AI) in Cystic Fibrosis Patients (AIR-CF3) C80117017 4 Examples of Active Grants Since 2006 PI Sponsor Grant # Start Stop Total Awarded to Date ______ 03/01/08 02/28/09 $20,000 Assay of Aminoglycosides in a CF Mouse Model Title BEDWELL, DAVID M TECHNION - ISRAEL INSTITUTE OF TECHNOLOGY CLANCY, JOHN PAUL KALOBIOS PHARMACEUTICALS, INC. KB001-03 03/01/08 02/28/09 $35,604 A Phase I/II Randomized, Double-Blind, Placebo-Controlled, Single Dose, Dose Escalation Study of KB001-Cystic Fibrosis Patients Infected with Pseudomonas Aeruginosa GUTIERREZ, HECTOR H UNIVERSITY OF MASSACHUSETTS (WORCHESTER) 337890 07/01/08 02/28/09 $4,000 The Effect of Formula Fortified with Docosahexaenoic Acid (DHA) on Infants with Cystic Fibrosis GUTIERREZ, HECTOR H Cystic Fibrosis Foundation Therapeutics, Inc./Children's Hospital & Regional Medical Center EPIC002 (SCHEDULE 4.0) 11/11/04 03/31/09 $65,649 The EPIC Observational Study: Longitudinal Assessment of Risk Factors for and Impact of Pseudomonas Aeruginosa Acquistion and Early Anti-Pseudomonal Treatment in Children with CF GUTIERREZ, HECTOR H CYSTIC FIBROSIS FOUNDATION THERAPEUTICS, INC. EPIC001 (SCHEDULE 3.0) 11/11/04 03/31/09 $40,357 Efficacy and Safety of Intermittent Animicrobial Therapy for the Treatment of the New Onset Pseudomonas Aeruginosa Airway Infection in Young Patients with Cystic Fibrosis CLANCY, JOHN PAUL CYSTIC FIBROSIS FOUNDATION CLANCY06A0 05/01/06 04/30/09 $390,494 Detection Of Airway CFTR Expression, Localization, and Activity In CF Patients. ROWE, STEVEN M NIH-NIDDK P30DK72482 05/01/07 04/30/09 $86,252 Pilot: Role of Flavonoids in CFTR Biogenesis and Activation VX06-770-101 05/04/07 05/03/09 $77,686 A Phase II study of VX-770 in CF Subjects harboring G551D and other surface localized CFTR mutations YOTHER, JANET L VERTEX PHARMACEUTICALS, INC. / PPD DEVELOPMENT, LLC. NIH-NHLBI T32HL07553 07/01/03 06/30/09 $1,671,051 Basic Mechanisms in Lung Diseases HAGOOD, JAMES HRSA T72MC00001 07/01/05 06/30/09 $1,352,427 Pediatric Pulmonary Center GUIMBELLOT, JENNIFER NIH-NIEMS F30ES14987 07/15/06 07/14/09 $96,762 BENOS, DALE J NIH-NIDDK R01DK37206 08/01/03 07/31/09 $1,678,397 CLANCY, JOHN PAUL TRANSAVE, INC. TR02-106 09/30/07 08/30/09 $73,023 Multidose Safety and Tolerability Study of Dose Escalation of Liposomal Amikacin for Inhalation (Arikace), in Cystic Fibrosis Patients GUTIERREZ, HECTOR H ALABAMA DEPARTMENT OF PUBLIC HEALTH C90118098 10/01/08 09/30/09 $68,379 Cystic Fibrosis Screening for Newborn Infants ROWE, STEVEN M 5 Effects of Ozone on CFTR Expression and HIF Signaling Sodium Entry Into Amiloride-Sensitive Epithelia Examples of Active Grants Since 2006 PI Sponsor Grant # Start Stop Total Awarded to Date Title MATALON, SADIS NIH-NHLBI R01HL75540 12/15/04 11/30/09 $1,406,668 Nitric Oxide Modulation of CFTR Expression and Function COLLAWN, JAMES F CYSTIC FIBROSIS FOUNDATION COLLAW08PO 12/01/08 11/30/09 $75,000 Cell Biology of CFTR in Polarized Epithelia CLANCY, JOHN PAUL INSPIRE PHARMACEUTICALS, INC 08-110 05/28/08 12/31/09 $55,685 A Phase 3, International, Multi-Center, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Efficacy and Safety Study of Denufosol Tetrasodium Inhalation Solution in Patients with Cystic Fibrosis Lung Disease and FEV1>= 75% Predicted GUTIERREZ, HECTOR H CYSTIC FIBROSIS FOUNDATION GUTIER09QI0 01/01/09 12/31/09 $27,000 Early Recognition and intervention for Acute Pulmonary Exacerbation in CF ROWE, STEVEN M VERTEX PHARMACEUTICALS, INC. BERDIEV, BAKHROM K UNIVERSITY OF ALABAMA HEALTH SERVICES FOUNDATION HARTMAN IV, JOHN L. COLUMBIA UNIVERSITY / CYSTIC FIBROSIS FOUNDATION SORSCHER, ERIC J NIH-NIDDK CLANCY, JOHN PAUL and ROWE, STEVEN M 01/01/09 12/31/09 $92,906 NPD Overreading Services for Clinical Trial Entitled "A Randomized, Double-Blind, Placebo-Controlled, Multiple Dose Study of VX-809 to Evaluate Safety, Pharmacokinetics, and Pharmacodynamics of VX-809 in Cystic Fibrosis Subjects Homozygous for the F508-CFTR Gene Mutation" ______ 03/01/07 02/28/10 $216,000 Fluorescence Lifetime-Resolved Imaging: Nanoscale Mapping of Dynamic Cellular Events (Pursues CFTR/ENaC Interactions) MILLER08 04/01/08 03/31/10 $94,400 Systematic Genetic and Biochemical Analysis of ER Quality Control (Designed to determine cellular targets for ΔF508 correction) P30DK72482 05/01/09 04/30/10 $66,510 UAB CF Research and Translation Core Center - NIDDK Equipment Supplement CYSTIC FIBROSIS FOUNDATION CLANCY05Y2 01/01/06 04/30/11 $642,688 UAB Cystic Fibrosis National NPD Reading Resource Center Core CLANCY, JOHN PAUL CYSTIC FIBROSIS FOUNDATION 41339119.032 06/01/09 05/31/10 $39,700 A Multi-Center, Phase IIB, Randomized, Placebo-Controlled, Double-Blind Study of The Effects of N-Acetylcysteine of Redox Changes and Lung Inflammation in Cystic Fibrosis Patients (NAC40630) PYLE, LOUISA NIH F30HL096389 06/22/09 06/21/11 $33,450 Potentiation of Mutant CFTR Activity WOODWORTH, BRADFORD AMERICAN ACADEMY OF OTOLARYNGOLOGY ______ 07/01/09 06/30/10 $25,000 Novel Flavonoid Compounds for Cystic Fibrosis Chronic Rhinosinusitis VX08-809-101 6 Examples of Active Grants Since 2006 Sponsor Grant # Start Stop Total Awarded to Date FU, LIANWU (KARL) AMERICAN LUNG ASSOCIATION ______ 07/01/08 06/30/10 $75,200 Regulation of the Degradation of Cystic Fibrosis Transmembrane Conductance Regulator under Cystic Fibrosis Conditions GUTIERREZ, HECTOR H Cystic Fibrosis Foundation C001-06 07/01/04 06/30/10 $773,166 Cystic Fibrosis Center for Care, Teaching and Research 6T72MC0000119-01 07/01/09 06/30/10 $332,095 Pediatric Pulmonary Center FREDER09B0 07/01/09 06/30/10 $48,000 CFF Clinical Fellowship PI CLANCY, JOHN PAUL ROWE, STEVEN M DHHS - HEALTH RESOURCES AND SERVICES ADMINISTRATION (MATERNAL AND CHILD HEALTH BUREAU) CYSTIC FIBROSIS FOUNDATION Title CLANCY, JOHN PAUL INSPIRE PHARMACEUTICALS, INC 08-114 07/13/09 07/12/10 $36,169 Open-Label Extension of Study 08-110 - A Multi-Center Study of Denufosol Tetrasodium Inhalation Solution in Patients with Cystic Fibrosis Lung Disease BENOS, DALE J NIH-NIDDK R56DK37206 08/17/09 07/31/10 $362,500 Sodium Entry Into Amiloride-Sensitive Epithelia BERDIEV, BAKHROM K NIH - NHLBI R21HL085112 09/01/08 07/31/10 $412,500 Regulation of Epithelial Sodium Channel by CFTR Chloride Channel DELUCAS, LAWRENCE J. CYSTIC FIBROSIS FOUNDATION DELUCA05XXO 08/01/05 07/31/12 $2,063,100 Crystallization and Structure Determination of Full-Length CFTR Protein ROWE, STEVEN M PTC THERAPEUTICS, INC. PTC124-GD-009CF 08/05/09 12/31/11 $243,755 Nasal PD Central Reading Services for Clinical Trial Entitled: A Phase 3 Efficacy and Safety Study of PTC124 as an Oral Treatment for Nonsense-Mutation-Mediated Cystic Fibrosis ROWE, STEVEN M CYSTIC FIBROSIS FOUNDATION ROWE08XX0 09/01/08 08/31/10 $25,000 CFTR Activity of Novel F508 Corrector Agents BROUILLETTE, CHRISTIE CYSTIC FIBROSIS FOUNDATION BROUIL08XXO 09/01/08 08/31/12 $175,755 Expression and purification of CFTR Domain Constructs CHUNG, WOOK JOON NIH-NINDS R21NS067693 09/25/09 08/31/10 $208,358 A Novel HTS Cell-Based Imaging Assay to Identify Chemical Correctors for F508-CFTR Defects SCHWIEBERT, ERIK M NIH-NIDDK R43 DK084658 09/30/09 08/31/10 $308,000 CF Corrector Ligands Discovered in CF Human Airway Cells DELUCAS, LAWRENCE J NIH - NIGMS R01GM081799 09/15/08 08/31/10 $869,148 Innovative Methods for Membrane Protein Expression and Crystallization ROWE, STEVEN M CYSTIC FIBROSIS FOUNDATION ROWE09CS0 10/01/09 09/30/10 $56,841 Special Consultant for Translational Science 7 Examples of Active Grants Since 2006 PI BROUILLETTE, CHRISTIE Sponsor CYSTIC FIBROSIS FOUNDATION Grant # BROUIL07XX0 Start Stop Total Awarded to Date 11/01/07 10/31/12 $561,202 Recombinant CFTR: Cooperativity of Structural Domains 01/01/08 12/31/10 $60,000 Matrix Metalloproteinase-9 in Pediatric RSV-Induced Respiratory Failure Title CLANCY, JOHN PAUL KAUL PEDIATRIC RESEARCH INSTITUTE ROWE, STEVEN M and YOUNG, K. RANDALL CYSTIC FIBROSIS FOUNDATION CLANCY09Y0 01/01/07 12/31/11 $1,021,368 ROWE, STEVEN M MASSACHUSETTS GENERAL HOSPITAL 323840 01/01/08 12/31/10 $31,900 Development of Optical Coherence Tomography for Measures of Mucociliary Clearance CLANCY, JOHN PAUL VERTEX PHARMACEUTICALS, INC. VX06-770-101 08/29/07 12/31/10 $47,940 Nasal PD Central Reading Services for Study Entitled: A Phase 2a, Randomized, Double-Blind, Placebo-Controlled Study of VX770 t R56DK056796 02/01/10 01/31/11 $219,750 New Paradigms of CFTR Regulation R01DK56796 09/01/05 01/31/11 $1,149,353 New Paradigms of CFTR Regulation ROWE09XX0 03/01/09 02/28/11 $50,000 Ussing Chamber Studies to Characterize CFTR Modulators 03/01/10 02/28/11 $20,000 Assay of Aminoglycosides in a CF Mouse Model KIRK, KEVIN L KIRK, KEVIN L NIH - NATIONAL INSTITUTE OF DIABETES & DIGESTIVE & KIDNEY DISEASES NIH-NIDDK ROWE, STEVEN M CYSTIC FIBROSIS FOUNDATION BEDWELL, DAVID M TECHNION - ISRAEL INSTITUTE OF TECHNOLOGY ______ ______ UAB Cystic Foundation Therapeutic Development Center VERTEX PHARMACEUTICALS, INC. VX08-809-101 03/01/09 02/28/11 $98,070 A Randomized, Double-Blind, Placebo-Controlled, Multiple Dose Study of VX-809 to Evaluate Safety, Pharmacokinetics, and Pharmacodynamics of VX-809 in Cystic Fibrosis Subjects Homozygous for the F508-CFTR Gene Mutation ROWE, STEVEN M PTC THERAPEUTICS, INC. PTC124-GD-009CF 03/01/09 02/28/11 $113,422 A Phase 3 Efficacy and Safety Study of PTC124 as an Oral Treatment for Nonsense-Mutation-Mediated Cystic Fibrosis GAGGAR, AMIT CYSTIC FIBROSIS FOUNDATION GAGGAR07AO 04/01/07 03/31/11 $254,604 Implications of cross-protease dysregulation in CF GILEAD SCIENCES, INC. EA-US-205-0111 05/21/08 05/20/11 $4,000 CLANCY, JOHN PAUL CLANCY, JOHN PAUL 8 Expanded Access Program for Aztreonam Lysine for Inhalation in Patients with Cystic Fibrosis and Pseudomonas Aeruginosa Airway Infection Who Have Limited Treatment Options and are at Risk for Disease Progression Examples of Active Grants Since 2006 PI Sponsor Grant # Start Stop Total Awarded to Date Title ROWE, STEVEN M NIH-NIDDK R03DK084110 08/25/09 06/30/11 $73,188 Mechanisms Underlying Protein Repair of CFTR Nonsense Mutations BLALOCK, J EDWIN NIH - NATIONAL HEART, LUNG, AND BLOOD INSTITUTE R01HL087824 07/01/09 06/30/11 $411,364 Therapeutics for Chronic Lung Disease: New Antagonists of PGP, Chemokines and CXCR GUTIERREZ, HECTOR H VERTEX PHARMACEUTICALS, INC. VX-08-770-103 07/10/09 07/09/11 $104,765 A Phase 3, 2-Part, Randomized, Double-Blind, PlaceboControlled, Parallel-Group Study to Evaluate the Pharmacokinetics, Efficacy and Safety of VX-770 in Subjects Aged 6 to 11Years with Cystic Fibrosis and the G551D Mutation ROWE, STEVEN M VERTEX PHARMACEUTICALS, INC. VX-08-770-102 07/10/09 07/09/11 $119,045 A Phase 3, Randomized, Double-Blind, Placebo Controlled, Parallel Group Study to Evaluate the Efficacy and Safety of VX 770 in Subjects with Cystic Fibrosis and the G551D Mutation STEELE, CHAD NIH-UNIVERSITY OF PITTSBURGH 08/10/09 07/31/11 $109,627 Immune Tolerance and Inflammation in ABPA in Patients with Cystic Fibrosis SORSCHER, ERIC J CYSTIC FIBROSIS FOUNDATION SORSCH05X0 09/01/06 08/31/12 $2,019,168 GUTIERREZ, HECTOR H NIH-NIDDK P30 DK072482 09/12/09 08/31/11 $204,401 UAB CF Research and Translation Core Center - Pilot Supplement ROWE, STEVEN M NIH-NCRR UL1RR025777 09/17/09 03/31/12 $500,000 UAB Center for Clinical and Translational Science (CCTS); (ARRA Supplement (to conduct a multicenter trial of quercetin as an activator of mutant CFTR) GUTIERREZ, HECTOR H ALABAMA DEPARTMENT OF PUBLIC HEALTH C00113045 10/01/09 09/30/11 $136,758 Cystic Fibrosis Screening for Newborn Infants BEDWELL, DAVID M NIH - NATIONAL INSTITUTE OF GENERAL MEDICAL SCIENCES R01GM06885407S1 12/01/07 11/30/11 $1,038,039 Mechanism of Eukaryotic Translation Termination (Basic studies to understand mechanism of CFTR truncation alleles) ______ New Reagents for Understanding and Overcoming the Delta-F508 Mutation CLANCY, JOHN PAUL KAUL PEDIATRIC RESEARCH INSTITUTE ______ 02/01/10 01/31/12 $50,000 Developing GI Outcome Measures for CFTR Modulator Trials in Young CF Patients HARRIS, WILLIAM THOMAS KAUL PEDIATRIC RESEARCH INSTITUTE ______ 02/01/10 01/31/12 $30,000 Myofibroblast proliferation in cystic fibrosis: a novel mechanism of disease modification 9 Examples of Active Grants Since 2006 PI Sponsor Start Stop Total Awarded to Date Title CF 2110399 02/08/10 02/07/12 $33,239 A Randomized, Double Blind, Parallel Group, Placebo Controlled 28 Day Study to Investigate the Safety, Tolerability and Pharmacodynamics of SB-656933 in Patients with Cystic Fibrosis (CF 2110399) ______ 03/01/09 02/28/12 awarded Bidirectional Transfer of Isolated Primary Airway Cells Obtained from Lung and Sinus Explants Grant # YOUNG, K. RANDALL GLAXOSMITHKLINE ROWE, STEVEN M VERTEX PHARMACEUTICALS, INC. BEDWELL, DAVID M NIH - NIDDK P30DK72482 05/01/07 04/30/12 $619,962 UAB CF Research and Translation Core Center - Mouse Models KIRK, KEVIN L NIH-NIDDK P30DK72482 05/01/07 04/30/12 $581,639 Core A: Cell Model and Assay Core SORSCHER, ERIC J NIH-NIDDK P30DK72482 05/01/07 04/30/12 $752,209 UAB CF Research and Translation Core Center - Administrative Core ROWE, STEVEN M NIH - NIDDK P30DK72482 05/01/07 04/30/12 $389,146 UAB CF Research and Translation Core Center - Clinical Core ROWE, STEVEN M CYSTIC FIBROSIS FOUNDATION CLANCY 05Y2 01/01/11 04/30/12 $224,062 UAB Center for CFTR Detection CORMET-BOYAKA, ESTELLE AMERICAN LUNG ASSOCIATION ______ 07/01/10 06/30/12 $160,000 Cadmium Alters Regulation of Lung Function via Ion Transport and Inflammatory Cytokines KIRK, KEVIN L NIH-NHLBI R01HL58341 07/01/07 06/30/12 $978,750 Regulation of CFTR by Syntaxin and N-sec 1 Isoforms CLINES, GREGORY A. NIH-NATIONAL INSTITUTE OF ARTHRITIS AND MUSCULOSKELETAL AND SKIN DISEASES R21 AR056826 07/01/10 06/30/12 $156,024 CF Bone Disease: Convergence of CFTR and PTH Signaling BLALOCK, J EDWIN ALBANY MEDICAL COLLEGE SC07-10-001 08/15/08 07/31/12 $188,500 MMP-9 Modulation as an Immunotherapeutic Intervention for Respiratory Infections BEBOK, ZSUZSANNA NIH-NHLBI R01HL76587 05/01/04 03/31/13 $1,894,192 CHRISTIAN, BECKY NIH-NCRR UL1RR025777 05/01/11 04/30/13 $30,000 Making Connections: Improving Quality of Life in Adolescents with Cystic Fibrosis PIAZZA, GARY CYSTIC FIBROSIS FOUNDATION R464-CR07 06/01/11 06/30/13 $72,000 Spectral domain optical coherence tomography for the functional characterization of ion transport modulators JACKSON, PATRICIA CYSTIC FIBROSIS FOUNDATION R464-CR07 07/01/11 06/30/13 $72,000 Modulation of Small Matrikines by Reactive Aldehydes in CF 10 CFTR Biogenesis and Function in Epithelia Examples of Active Grants Since 2006 PI Sponsor Grant # Start Stop Total Awarded to Date Title CYSTIC FIBROSIS FOUNDATION R464-CR07 07/01/11 06/30/13 $72,000 Analysis of CFTR Post Translational Modification by Mass Spectrometry CHILDREN'S HOSPITAL & REGIONAL MEDICAL CENTER 346609 07/01/08 12/31/13 $9,164 CF Therapeutics Development Network Individual Project Schedule #6.0 BLALOCK, J. EDWIN NIH-NHLBI R01HL077783 04/01/06 03/31/14 $1,789,648 GUTIERREZ, HECTOR H CHILDREN'S HOSPITAL & REGIONAL MEDICAL CENTER 41339135.032O 04/01/09 03/31/14 $17,694 COLLAWN, JAMES F NIH-NIDDK R01DK60065 12/20/02 04/30/14 $1,790,554 ABRAHAM, EDWARD NIH-NHLBI R01GM087748 06/01/09 05/31/14 $303,642 WANG, GUANGYU AMERICAN HEART ASSOCIATION ______ 07/01/10 06/30/14 $77,000 ______ 12/01/07 12/01/14 $150,000 UAHSF General Endowment Fund Scholar Award for Studies of CFTR in Sinonasal Epithelial Cells R01GM095639 09/30/10 07/31/14 $300,000 Production & Crystallization of Membrane Protein for 3D Structure (includes studies of CFTR) BARNES, STEPHEN CLANCY, JOHN PAUL WOODWORTH, BRADFORD DELUCAS, LAWRENCE J UNIVERSITY OF ALABAMA HEALTH SERVICES FOUNDATION NIH - NATIONAL INSTITUTE OF GENERAL MEDICAL SCIENCES A New Pathway for Neutrophil-Induced Airway Inflammation The EPIC Observational Study: Longitudinal Assessment of Risk Factors for and Impact of Pseudomonas Aeruginosa Acquisition and Early Anti-Pseudomonal Treatment in Children with CF Cell Biology of CFTR in Polarized Epithelia HMGB1 and Neutrophil Efferocytosis Inhibition Mechanisms of the Regulatory Domain in the Cystic Fibrosis Transmembrane Conductance Regulator KIRK, KEVIN L NIH - NATIONAL INSTITUTE OF DIABETES & DIGESTIVE & KIDNEY DISEASES R01DK056796 02/01/11 01/31/15 $318,638 New Paradigms of CFTR Regulation GAGGAR, AMIT NIH-NHLBI R01HL102371 07/01/10 05/31/15 $377,902 A Novel Proteolytic System of Pulmonary Inflammation. HOOVER, WYNTON C DHHS - HEALTH RESOURCES AND SERVICES ADMINISTRATION (MATERNAL AND CHILD HEALTH BUREAU) 07/01/10 06/30/15 $365,000 UAB Pediatric Pulmonary Center T72MC00001 11 Examples of Active Grants Since 2006 PI Sponsor Grant # Start Stop Total Awarded to Date Title SORSCHER, ERIC J CYSTIC FIBROSIS FOUNDATION R464-CR11 07/01/11 06/30/15 $284,000 Research Development Program - Component II FULLER, CATHERINE M NIH-NIDDK R01DK037206 08/01/10 07/31/15 $366,250 Sodium Entry into Amiloride-Sensitive Epithelia ROWE, STEVEN M NIH/NATIONAL HEART, LUNG, BLOOD INSTITUTE R01HL405487 02/15/11 01/31/16 $367,550 Molecular Pathogenesis and Phenotype of Acquired CFTR dysfunction in COPD WOODWORTH, BRADFORD NIH - NATIONAL INSTITUTE OF DENTAL & CRANIOFACIAL RESEARCH K08DE021426 03/01/11 02/29/16 $133,596 Chloride Secretagogues for Acquired CFTR Dysfunction in Chronic Rhinosinusitis BEDWELL, DAVID M TECHNION - ISRAEL INSTITUTE OF TECHNOLOGY 04/01/11 03/31/16 $134,677 Tuning aminoglycosides for treatment of genetic diseases R01 GM094792 (subcontract) Total for the above awarded grants = consists of: NIH funding = CFF/CFFT funding = All other sponsors = Anesthesiology Cell Biology Chemistry Genetics Medicine Microbiology Neurobiology $28,814,579 $10,001,238 $7,218,060 $46,033,877 $46,033,877 62.59% 21.73% 15.68% 100.00% Interdisciplinary Departments represented by the above Principle Investigators: (Ji, Matalon) (Bebok, Berdiev, Collawn, Fu, Sztul) (Brouillette) (Guimbellot, Hartman, Pyle) (Blalock, Clines, Gaggar, Liu, Rowe, Sorscher, Steele) (Bedwell, Yother) (Chung) Nursing (Christian) Optometry (Delucas) Pediatrics Pharmacology Physiology & Biophysics (Clancy, Gutierrez, Harris) (Falany) (Benos, Kirk, Schwiebert) Public Health (Fannuchi) Surgery (Woodworth) Updated 2nd Quarter, 2011 12