Thermodynamics and phase diagrams in metallurgy: no
advertisement

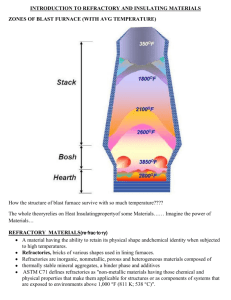
Thermodynamics and phase diagrams in metallurgy: no more secrets Agoria together with InsPyro organizes a four day course program on thermodynamics and phase diagrams in metallurgical processes. The course will be given in English and aims to attract material scientists who want a refreshment of the metallurgical principles, and hands-on metallurgists who wish to strengthen their knowledge of the theoretical background. The first day (01/04/2014) aims to explain the theoretical background of thermodynamics in relation to an industrial reality. The visualization of equilibria and the interpretation of phase diagrams is tackled on the second day (06/05/2014). This is combined with a course day dedicated to the microstructure formation of iron and steel castings (07/05/2014). During the last day the focus will be set on refractory materials and their properties (03/06/2014). More detailed information on the content can be found in the day programs. 01/04/2014: Introduction to thermodynamics: from theory to practice (location: Agoria, Brussel) 06/05/2014: How to read a phase diagram (location: Agoria, Brussel) 07/05/2014: Thermodynamics and phase diagrams for iron and steel processing (location Agoria, Brussel) 03/06/2014: Refractory (location: Agoria, Brussel) Price 450€/course day (for Agoria-members and InsPyro contacts) 675€/ course day (non-members) or 1620€/full course program (4 days) (for Agoria-members and InsPyro contacts) 2430€/full course program (4 days) (non-members) 1. Introduction to thermodynamics: from theory to practice (01/04/2014) What is the idea behind thermodynamics? Materials change (concepts: component, phases, mixtures, activities) and materials react (concepts: enthalpy, equilibrium, solubility). How they change and react can be calculated based on thermodynamic principles which determine how reactions proceed, how processes behave and how a final structure looks like. Starting from the laws of thermodynamics, phase stability is explained. Important concepts as Gibbs free energy, enthalpy, entropy, phase rule of Gibbs are discussed. The link to important properties in metallurgy, such as solubility, melting point, and precipitation is made. The course provides both the mathematical formulae as well as it attempts to create a feeling of ‘common sense’ principles derived from the basic laws. During the last hour of this course industrial examples are tackled to establish the connection between industrial reality and the theory of thermodynamics. 09.30: Registration 10.00: What is the idea behind thermodynamics? Materials change (concepts: component, phase, mixture, activity) Materials react (concepts: enthalpy, equilibrium, solubility) 12.00: Thermodynamics to evaluate reactions and processes 13.00: Sandwich lunch 14.00: Calculation of phase diagrams 15.30: Thermodynamics to construct phase diagrams Change of phase fractions as a function of a process parameter Change of composition of a phase as a function of a process parameter Change of melting temperature as a function of composition 16.00: Industrial examples - Heat balance — Addition of scrap to liquid steel, lead, or copper - Effect of pressure on the degradation of graphite or carbon containing crucibles - Slag-refractory interaction — Mullite refractory, MgO-C refractory - Exploring feasibility — Can we reduce Al2O3 with C? Can we make nickel from stainless steel? - … — Your suggestions! 17.00: Opportunity for discussion 2. How to read a phase diagram? (06/05/2014) Phase diagrams are graphical representations of materials, most commonly as a function of temperature and composition. In this "how to read a phase diagram" course the main focus is on the practical aspects of reading. It gives an insight into the type of information you can retrieve from a phase diagram and how to give an interpretation to the different lines and areas in the graph. The course starts with a basic level introduction but rapidly advances to more complex systems. Exercises are foreseen to ensure a thorough understanding. Exercises in this general part focus on all kinds of material systems (molten metals, slags, solid phase formation etc.). A next course focuses on specific diagrams and mechanisms for cast iron and steel. 09.30: Registration 10.00: Binary diagrams Different areas and lines in a binary phase diagram Lever rule to determine phase fractions and phase composition Eutectic, peritectic, eutectoid, peritectoid Microstructure formation upon equilibrium cooling 11.30: Ternary diagrams Interpretation of a liquidus projection Isothermal section to see the solubility in the liquid or matrix phase Lever rule to determine phase fractions and phase compositions Reaction between liquid and solid (e.g. refractory) 13.00: Sandwich lunch 14.00: Binary diagrams Microstructure formation in non-equilibrium cooling conditions Miscibility gap 15.30: Ternary diagrams Different areas and lines in a ternary phase diagram Horizontal sections (isothermal) and vertical sections of a ternary diagram Determine phase fractions and phase compositions Eutectic and peritectic reaction Miscibility gap Phase formation upon cooling (basic phase reactions) 16.30: Quaternary and higher order diagrams 17.00: Opportunity for discussion 3. Phase diagrams and microstructure formation for iron and steel processing (07/05/2014) This session has its focus on phase diagrams for iron and steel industry. Starting from the Fe-C/ Fe-Fe3C diagram the basic principles are rehearsed and then expanded to the influence of alloying elements. Also the link between phase diagram and microstructure formation is made. 09.30: Registration 10.00: Basic phase diagram Fe-C: white cast iron zone Fe–Fe3C: grey cast iron zone and carbon steel zone 10.30: Different phases, different structure, different properties FCC-BCC-HCP 11.00: Microstructure formation in equilibrium Solidification and solidification path Heat treatment 13.00: Sandwich lunch 14.00: Alloying influences the phase stability: Fe-C-Ni, Fe-C-Cr,… FCC stabilization BCC stabilization Carbides: appearance and preferential formation with alloying elements Steel types (austenitic, martensitic, ferritic, TWIN, TRIP, Duplex,…) Cast iron types (white, grey, high-alloyed) 15.00: Microstructure formation outside equilibrium CCT and TTT diagrams (non-equilibrium cooling) Solidification path (Scheil assumptions) Some remarks on nucleation and growth 16.00: Oxide stability and formation of inclusions (Ellingham) Deoxidation equilibrium in steel and cast iron Reoxidation of elements in steel 17.00: Opportunity for discussion 4. Refractories in metallurgy: introduction to types and wear mechanisms (03/06/2014) A high performance is expected of refractories in the metal industry, especially for the consumable linings in furnaces and ladles. They are exposed to liquid materials at high temperature and have to withstand tough conditions. This course aims to give an understanding of the behavior of the refractories while the furnace is in operation. This course should give you sufficient theoretical background to discuss with suppliers on possible improvements or to compare refractory datasheets. An overview of refractory materials is given with a focus on applicability in metallurgy, especially in the areas of the audience. Then, the possible wear mechanisms occurring in high-temperature furnaces and ladles are explained. The link is made to what is observed in analysis of damaged (“post-mortem”) linings and bricks. Participants are encouraged to send in questions for discussion or bring demo materials (a selection may need to be made). 09.30: Registration 10.00: What is a refractory? Boundary conditions for a refractory Oxides and other compounds and their high temperature resistance 10.30: Production of refractories Raw materials found in nature Processes for upgrading and binding 11.00: Refractories in metallurgy Overview of different types of refractories from different raw materials and processing, examples of datasheets Advantages and limitations of the different types Examples of suppliers Different wear mechanisms affecting refractories in general 13.00: Sandwich lunch 14.00: Refractory wear in furnaces and ladles What are normal wear mechanisms and lifetime in metallurgy Observations of accelerated wear Wear testing in lab and industrial scale 15.00: Analysis of a worn refractory lining and bricks What can be observed in post mortem analysis Clues for determining the wear mechanism 16.00: Opportunity for discussion