Topic 6b using moles (solutions)
advertisement
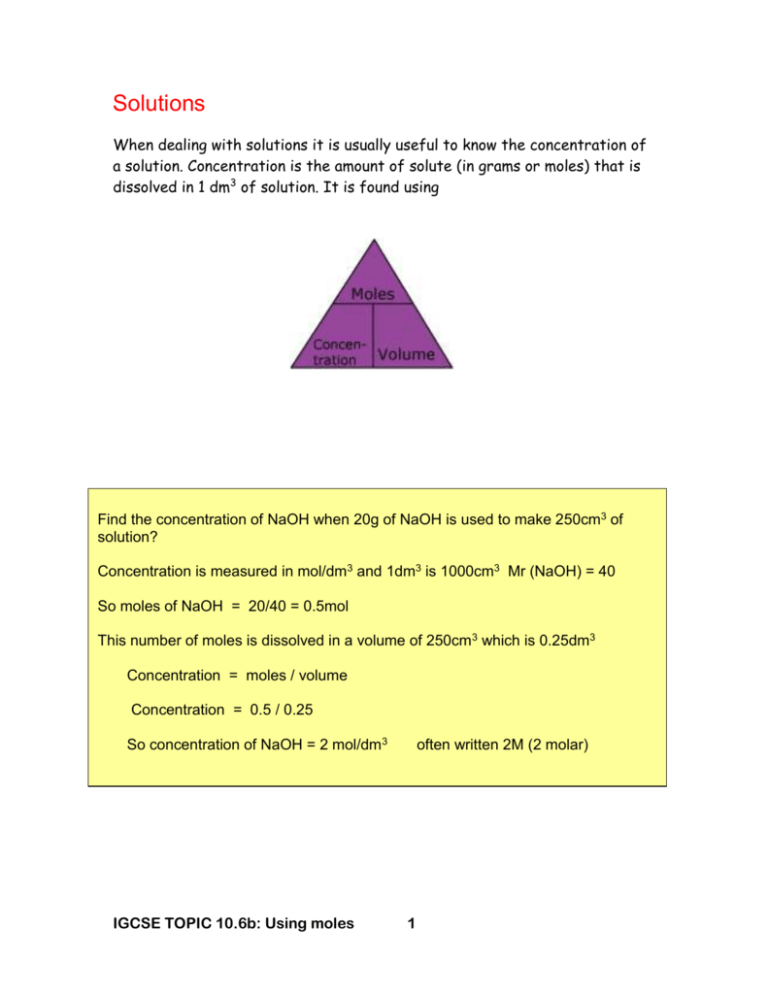
Solutions When dealing with solutions it is usually useful to know the concentration of a solution. Concentration is the amount of solute (in grams or moles) that is dissolved in 1 dm3 of solution. It is found using Find the concentration of NaOH when 20g of NaOH is used to make 250cm3 of solution? Concentration is measured in mol/dm3 and 1dm3 is 1000cm3 Mr (NaOH) = 40 So moles of NaOH = 20/40 = 0.5mol This number of moles is dissolved in a volume of 250cm3 which is 0.25dm3 Concentration = moles / volume Concentration = 0.5 / 0.25 So concentration of NaOH = 2 mol/dm3 IGCSE TOPIC 10.6b: Using moles often written 2M (2 molar) 1 CONCENTRATION OF SOLUTIONS We have already seen that we can use titration to prepare a sample of a salt. Titration is more often used to determine the concentration of an unknown solution. hydrochloric acid + sodium hydroxide HCl(aq) + NaOH(aq) sodium chloride + water. NaCl(aq) + H2O(l) The burette is filled with hydrochloric acid of known concentration. A known quantity of alkali (say 25 cm3) of sodium hydroxide of unknown concentration is added from a pipette into a conical flask. The tap on the burette is turned open to allow the acid to be added drop by drop into the alkali. The alkali contains an indicator (phenolphthalein) which is pink in an alkali and colourless in an acid. The flask is swirled during the experiment to ensure mixing. When enough acid has been added to neutralise the alkali the indicator changes from pink to colourless. This is called the end point and could also be determined using a pH meter. The titration can be repeated using the same amounts of acid and alkali and an average value obtained. IGCSE TOPIC 10.6b: Using moles 2 Other indicators can be used too. Methyl orange is red in acid and yellow in alkali, whilst litmus is red in acid and blue in alkali. Titration calculations 25cm3 of NaOH solution was exactly neutralised in a titration by 20.0cm 3 of 0.5mol/dm3 hydrochloric acid. Calculate the concentration of NaOH solution. The balanced equation is HCl + NaOH NaCl + H2O The equation tells us that 1 mole of NaOH react with 1 mole of HCl No of moles of HCl = concentration (mol/dm3) x volume (dm3) No of moles of HCl = = 0.5 x 20/1000 (dm3) 0.01 So in 20cm3 there are 0.01 moles of acid Remember that in this case there are 1 moles of NaOH reacting with 1 mole of HCl ie 1:1 In that case the 0.01 moles of acid must have reacted with 0.01 moles of NaOH 0.01 moles of NaOH was in 25cm3 (or 25/1000dm3) Therefore the concentration of the NaOH, in mol/dm3 = moles÷volume (dm3) So the concentration of the NaOH = 0.01 ÷ 25/1000 Or 0.40 mol/dm3 IGCSE TOPIC 10.6b: Using moles 3








