Worksheet #8: Reaction Classifications, Radical and Polar Reactions
advertisement
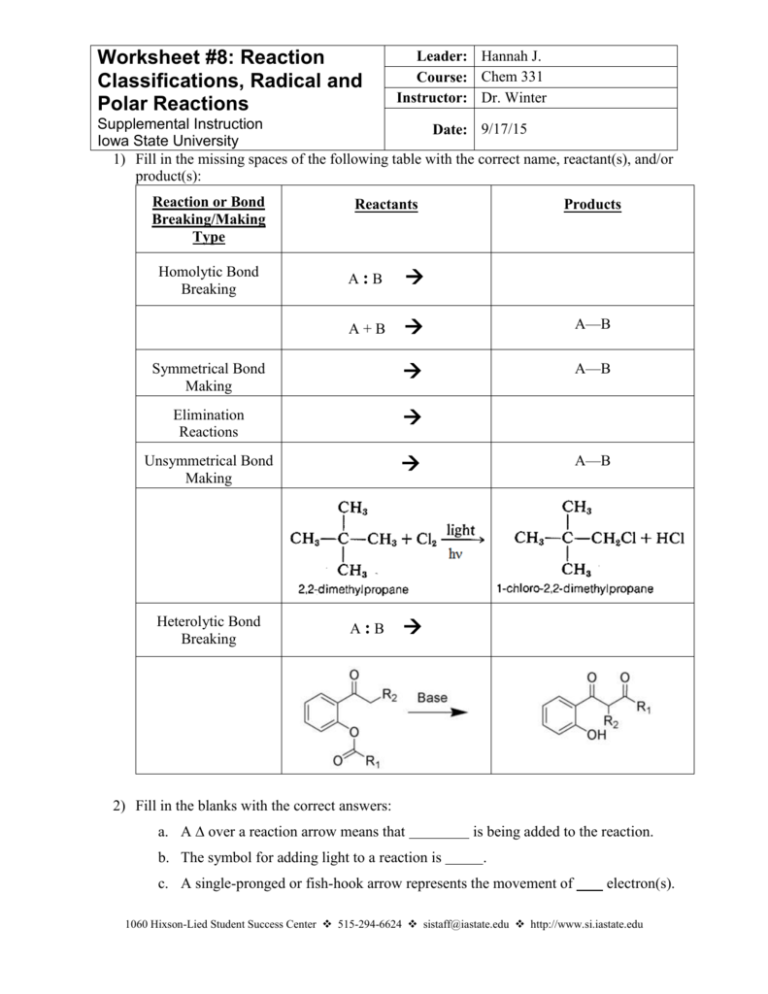
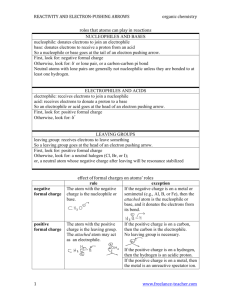
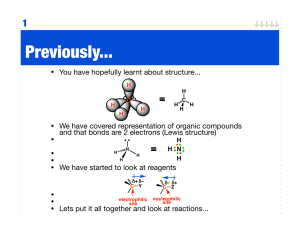
Worksheet #8: Reaction Classifications, Radical and Polar Reactions Leader: Hannah J. Course: Chem 331 Instructor: Dr. Winter Supplemental Instruction Date: 9/17/15 Iowa State University 1) Fill in the missing spaces of the following table with the correct name, reactant(s), and/or product(s): Reaction or Bond Breaking/Making Type Reactants Products A:B A+B A—B Symmetrical Bond Making A—B Elimination Reactions Unsymmetrical Bond Making Homolytic Bond Breaking Heterolytic Bond Breaking A:B A—B 2) Fill in the blanks with the correct answers: a. A ∆ over a reaction arrow means that b. The symbol for adding light to a reaction is is being added to the reaction. . c. A single-pronged or fish-hook arrow represents the movement of electron(s). 1060 Hixson-Lied Student Success Center 515-294-6624 sistaff@iastate.edu http://www.si.iastate.edu d. A arrow represents the movement of 2 electrons (a lone pair). e. A(n) (or Lewis base) is a nucleus-lover and, thus, is in electrons. f. A(n) (or Lewis acid) is an electron-lover and, thus, is in electrons. g. The always attacks the . 3) For the following reaction, the chlorination of methane, give the mechanism of the initiation and propagation steps(s) as well as a possible termination step, including arrow pushing in each mechanism. Chlorination of Methane: Initiation: Propagation: Termination: