water samples

Graham Co. Dept of Public Health Environmental Division
WATER SAMPLES - FEES AND PROCEDURES
Water samples can be obtained from the Graham County Environmental Health Division by visiting the Dept of Public
Health, at 21 south Main Street in Robbinsville, to submit an application and pay the fee of $20.00 and/or mailing the completed form to the Environmental Health Division at 21 South Main Street, Robbinsville, NC 28658, together with the applicable fee listed. The environmental teams have their certification lab status and now can do some testing in house.
Water samples are taken Monday through Thursday for the bacteriological.
Bacteriological - Samples are examined for the presence of the coliform group of bacteria, which are indicators of fecal contamination. Water is not examined for pathogenic bacteria, as the prospect of isolating them from water is very remote. Open wells, unprotected springs, or any water source with visible evidence of contamination are unsafe for drinking purposes, regardless of laboratory findings.
Here are other test that can be obtains and sent to the state lab
Inorganic Chemicals - These samples are routinely analyzed for alkalinity, arsenic, calcium, chloride, copper, hardness, lead, iron, magnesium, manganese, pH, fluoride, and zinc.
Nitrates/Nitrites - These samples also fall under the category of inorganic chemicals, but we can also sample for these separately. Nitrates/nitrites can be found in areas near hog farms, chicken houses, or heavy fertilization.
Petroleum/VOCs - Petroleum products fall into two categories: 1) solvents and gasoline; and 2) heavy oils and greases. If the suspected petroleum contaminant is a solvent or gasoline, request a Volatile Organic Compound
(VOC) sample. If the suspected contaminant is a heavy oil or grease, request a Petroleum sample.
Pesticides/Herbicides - Pesticide sampling may be important in areas where someone is applying products to kill pests, such as rodents, mites, mosquitoes, etc. Herbicide sampling may be important in areas where someone is applying products to kill weeds and other plants that grow where they are not wanted.
Sulfur/sulfate-reducing bacteria - Generally only important when you smell a "rotten egg" odor.
To learn more about contaminants commonly found in drinking water
Contact the Environmental Health Specialist 828-479-6232 or 828-479-7900 between 8 am and 5 pm after the application has been submitted to schedule a day for the sample to be taken.
Bacteriological sample results take a best 24 hours, chemical sample results take 3-4 weeks and all other sample results take 1-2 months.
Keep a copy of your completed application until the process is complete.
Interpreting Water Sample Results
If you are experiencing water quality issues with your well, we strongly advise contacting a water treatment specialist or a
Certified
Well Contractor
.
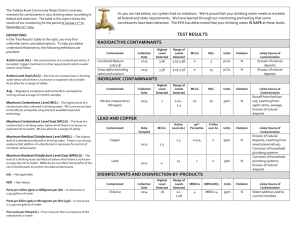
Most of the recommended limits or maximum contaminant levels (MCLs) for inorganics in public water supplies are as follows:
Antimony - MCL = 0.006 mg/L. May decrease growth and longevity. Potential sources are industrial discharges or from tin/antimony solder used in plumbing.
Arsenic - MCL = 0.010mg/L. Carcinogenic properties have been ascribed to arsenic. Its presence may be due to natural deposits, industrial discharges or pesticides.
Barium - MCL = 2 mg/L. Occurs only in trace amounts in drinking water and rarely exceeds 1 mg/L.
Beryllium - MCL = 0.004 mg/L. Beryllium is very poisonous. It is used in atomic reactors, aircraft, rockets and missile fuels. It is through discharges of such industries that it may enter water.
Cadmium - MCL = 0.005 mg/L. Cadmium is toxic and may be carcinogenic. It may enter water as a result of industrial pollution or
deterioration of galvanized pipe.
Chloride - MCL = 250 mg/L. High chloride levels may harm pipes, as well as impart an unpleasant salty taste.
Chromium - MCL = 0.10 mg/L. Chromium salts are used in industrial processes and may enter a water supply through industrial discharge.
Copper - MCL = 1.3 mg/L. It may impart a metallic taste to water and cause greenish stains on faucets and plumbing fixtures.
Cyanide - MCL = 0.2 mg/L. Can cause spleen, brain and liver damage. It is used in electroplating, steel processing, plastics, synthetic fibers, fertilizer and farm products.
Fluoride - MCL = 4.0 mg/L. Fluorides are found mostly in groundwater as a natural constituent.
Iron - MCL = 0.3 mg/L. Iron in water can cause staining of laundry and porcelain. It may give the water an astringent taste.
Lead - MCL = 0.015 mg/L. Lead is a cumulative poison. In a water supply it may occur where piping material or pipe joint compound
contains lead.
Manganese - MCL = 0.05 mg/L. Manganese can cause objectionable stains to laundry and fixtures.
Mercury - MCL = 0.002 mg/L. Mercury is very toxic and its presence may be associated with industrial water and agricultural applications.
Nitrate - MCL = 10 mg/L (as nitrogen). Serious poisonings in infants have occurred following ingestion of well water containing nitrate nitrogen at concentrations greater than 10 mg/L. This problem is known as methemoglobinemia and is generally confined to infants less than three months old. The presence of nitrates is usually due to animal wastes and fertilizers. Boiling water does not remove nitrates but instead concentrates them.
Nitrite - MCL = 1mg/L (as nitrogen). Nitrite is the actual etiologic agent of methemoglobenemia. It results from oxidation of ammonia or reduction of nitrates. May occur in natural water or water distribution systems.
Nickel - MCL = 0.1 mg/L. May affect the heart and liver. Can enter water supplies through discharges from battery, ceramics, or glass production.
pH - MCL = 6.5 - 8.5. A soft acid water may leach metals from plumbing causing staining problems, metallic tastes or deleterious health effects.
Selenium - MCL = 0.05 mg/L. It is an essential trace nutrient, but may be toxic above trace levels. Natural levels in groundwater may be due to soil types. Selenium may be leached from coal ash and fly ash at electric power plants that burn seleniferous coal.
Silver - MCL = 0.10 mg/L. Exposure to silver in drinking water may cause argyria (a discoloration of the skin). Health effects are only cosmetic.
Sulfate - MCL = 250 mg/L. May naturally be present in groundwater. Its sodium and magnesium salts exert a cathartic action.
Thallium - MCL = 0.002 mg/L. Affects the brain, kidneys, and liver. Its presence may be associated with electronics or glass industries.
Total Dissolved Solids - MCL = 500 mg/L. Waters with high dissolved solids are unpalatable and may be unsuitable for many industrial applications.
Zinc - MCL = 5 mg/L. Zinc may cause a bitter astringent taste and opalescence in alkaline water. Most often enters the water supply through the deterioration of galvanized iron pipes.
You may also click here
for the Environmental Protection Agency's (EPA's) National Primary Drinking Water Regulations for inorganics. For the EPA's National Secondary Drinking Water Regulations, click here
.