Classifying Chemical Reactions
advertisement
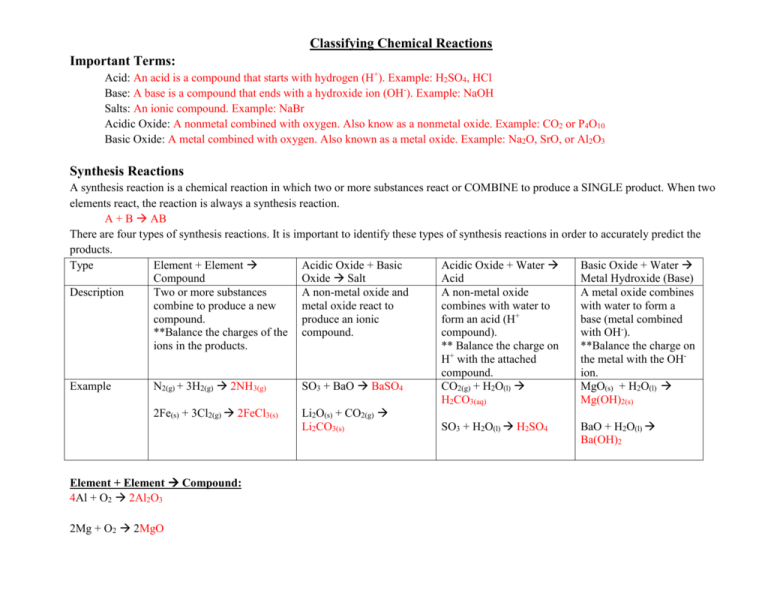
Classifying Chemical Reactions Important Terms: Acid: An acid is a compound that starts with hydrogen (H+). Example: H2SO4, HCl Base: A base is a compound that ends with a hydroxide ion (OH-). Example: NaOH Salts: An ionic compound. Example: NaBr Acidic Oxide: A nonmetal combined with oxygen. Also know as a nonmetal oxide. Example: CO2 or P4O10 Basic Oxide: A metal combined with oxygen. Also known as a metal oxide. Example: Na2O, SrO, or Al2O3 Synthesis Reactions A synthesis reaction is a chemical reaction in which two or more substances react or COMBINE to produce a SINGLE product. When two elements react, the reaction is always a synthesis reaction. A + B AB There are four types of synthesis reactions. It is important to identify these types of synthesis reactions in order to accurately predict the products. Type Element + Element Acidic Oxide + Basic Acidic Oxide + Water Basic Oxide + Water Compound Oxide Salt Acid Metal Hydroxide (Base) Description Two or more substances A non-metal oxide and A non-metal oxide A metal oxide combines combine to produce a new metal oxide react to combines with water to with water to form a + compound. produce an ionic form an acid (H base (metal combined **Balance the charges of the compound. compound). with OH-). ions in the products. ** Balance the charge on **Balance the charge on H+ with the attached the metal with the OHcompound. ion. Example N2(g) + 3H2(g) 2NH3(g) SO3 + BaO BaSO4 CO2(g) + H2O(l) MgO(s) + H2O(l) H2CO3(aq) Mg(OH)2(s) 2Fe(s) + 3Cl2(g) 2FeCl3(s) Li2O(s) + CO2(g) Li2CO3(s) SO3 + H2O(l) H2SO4 BaO + H2O(l) Ba(OH)2 Element + Element Compound: 4Al + O2 2Al2O3 2Mg + O2 2MgO Acidic Oxide + Basic Oxide Salt Practice: CO2 + CaO CaCO3 SO3 + Li2O Li2SO4 Acidic Oxide + Water Acid: SO3 + H2O(l) H2SO4 Basic Oxide + Water Metal Hydroxide (Base): Li2O + H2O(l) 2LiOH Al2O3 + 3H2O(l) 2Al(OH)3 Cu2O + H2O(l) 2CuOH CuO + H2O(l) Cu(OH)2 Decomposition Reactions The reverse of synthesis reactions, a decomposition reaction is one in which a single compound breaks down into two or more elements or new compounds. This is usually due to the addition of heat, light, or electricity. In generic terms, decomposition reactions look like the following: AB A + B Type Compound Element + Decomposition of salts Decomposition of Acids Decomposition of Bases Element Description A compound decomposes Several salts can be Acids can be Most bases decompose into two different elements. decomposed to form a basic decomposed into water to form water and a ** Remember diatomic oxide and an acidic oxide. and acidic oxide. basic oxide. elements occur in twos. Example 2HgO(s) 2Hg(l) + O2(g) Carbonates H2SO4(aq) H2O(l) + Ca(OH)2(s) H2O(l) + CaCO3(s) CaO(s) + CO2(g) SO3(g) CaO(s) Sulfates ZnSO4(s) ZnO(s) + SO3(g) Chlorates 2KClO3(s) 2KCl(s) + 3O2(g) ** A hydrate decomposes into the compound and water. Practice: o 2NaClO3 2NaCl + 3O2 o 2AgI 2Ag + I2 o Cs2CO3 Cs2O + CO2 o Ni(ClO3)2 NiCl2 + 3O2 o Ni(OH)2 H2O + NiO o 2AlF3 2Al + 3F2 COMPLETE QUESTION 3 ON BALANCING EQUATION SHEET AND IDENTIFY AS A SYNTHESIS OR DECOMPOSITION EQUATION. IF SYNTHESIS, IDENTIFY THE TYPE. Combustion Reactions In a combustion reaction, oxygen combines with a substance and releases energy in the form of heat and light. A combustion reaction will be in the form: FUEL + O2(g) Common Oxide of the fuel If the fuel contains … then a product is… o C CO2 o H H2O o N NO2 o S SO2 ALSO: CwHxOySz + O2(g) CO2(g) + H2O(g) **A combustion reaction needs oxygen gas O2(g) and a fuel!!!** Example: C3H8 (s) + O2(g) 3CO2(g) + 4H2O(g) a) b) c) d) e) Practice: Complete the combustion reactions for the following organic molecules. C2H6 f) C2H6O C3H8 g) C6H8O6 C3H6O3 h) C2H4O2 C13H28 i) C22H46 C2H6O2 j) C36H74 k) l) m) n) o) C8H20 C5H10O5 C20H30O CH4O C4H8O4 Replacement Reactions In contrast to synthesis, combustion, and decomposition reactions, many chemical reactions involve the replacement of an element in a compound. There are two types of replacement reactions: singlereplacement and double-replacement. Single Replacement Reactions Single - replacement reactions are those in which the atoms of one element replace the atoms of another element in a compound. Something is passes from one group to another much like someone cutting in on a dance. The person who was initially standing alone is now dancing in a partnership while someone who had been dancing is now by themselves. This is the form of A + BX AX + B Type Metal + water Metal + Ionic Compound Description A metal and water react and the metal replaces a hydrogen atom in a water molecule. A metal and an ionic compound react and the metal will replace the metal in the compound. Example 2Li (s) + 2H2O(l) 2LiOH(aq) + H2(g) Cu(s) + 2 AgNO3(aq) 2Ag(s) + Cu(NO3)2 Non-metal + Ionic Compound A non-metal and an ionic compound will react and the non-metal will replace the nonmetal in the compound. Halogens are frequently involved in these reactions. F2(g) + 2 NaBr(aq) 2NaF(aq) + Br2(l) Metal atoms might replace another metal, depending on their reactivities. A metal’s reactivity is its ability to react with another substance. This activity series orders metals by their reactivity with other metals. The activity series table will help up predict whether a chemical reaction will occur or not. Cu(s) + 2 AgNO3(aq) 2Ag(s) + Cu(NO3)2 Single – replacement reactions like the one between copper and aqueous silver nitrate determines a metal’s position on the list. The most active metals, which are those that do replace the metal in a compound, are at the top of the list. The least active metals are at the bottom. **A specific metal cannot replace any metal listed above it. For example, copper atoms replace silver atoms in a solution of silver nitrate. However, if you place a silver wire in aqueous copper(II) nitrate, the silver atoms will not replace the copper. Silver is listed below copper in the activity series and no reaction occurs. The letters NR (no reaction) are commonly used to indicate that a reaction will not occur. Ag(s) +Cu(NO3)2(aq) NR Similar to metals, halogens exhibit different activity levels in single – replacement reactions. A more reactive halogen replaces a less reactive halogen that is part of a compound dissolved in water. Br2(g) + 2NaF(aq) NR Practice: Fe(s) + CuSO4(aq) FeSO4(aq) + Cu(s) Br2(l) + MgCl2(aq) NR 6H2O(l) + 2Al(s) 3H2(g) + 2Al(OH)3(s) 3Mg(s) + 2AlCl3(aq) 3MgCl2(aq) + 2Al(s) 2K(s) + ZnCl2(aq) 2KCl(aq) + Zn(s) Cl2(g) + HF(aq) NR Fe(s) + Na3PO4(aq) NR Double – Replacement Reactions A double-replacement reaction involves an exchange of ions between two compounds. Thinking back to the dance analogy, two couples dancing together when they decide to switch partner with one another. AX + BY AY + BX In this generic equation, A and B represent positively charged ions (cations), and X and Y represent negatively charged ions (anions). You can see that the anions have switched places and are now bonded to the other cations in the reaction. In other words, X replaces Y and Y replaces X. Step 1. 2. 3. 4. 5. 6. 7. Guidelines for Double-Replacement Reactions Example Write the components of the reactants in a Ca(OH)2(aq) + HCl(aq) skeleton equation. Identify the cations and anions in each Ca(OH)2 has Ca2+ and OHcompound. HCl has H+ and ClPair up each cation with the anion from the Ca2+ pairs with Clother compound. H+ and OHWrite the formulas for the products using CaCl2 the pairs from step 3. H2O Write the complete equation for the double- Ca(OH)2(aq) + HCl(aq) CaCl2 + H2O replacement reaction. Use the solubility chart to predict the states Ca(OH)2(aq) + HCl(aq) CaCl2(aq) + H2O(l) of the products. Balance the equation. Ca(OH)2(aq) + 2HCl(aq) CaCl2(aq) + 2H2O(l) **Note: A solid produced during a chemical reaction in a solution is called a precipitate. Example: 2NaOH(aq) + CuCl2(aq) 2NaCl(aq) + Cu(OH)2(s)









