Analysis testans
advertisement
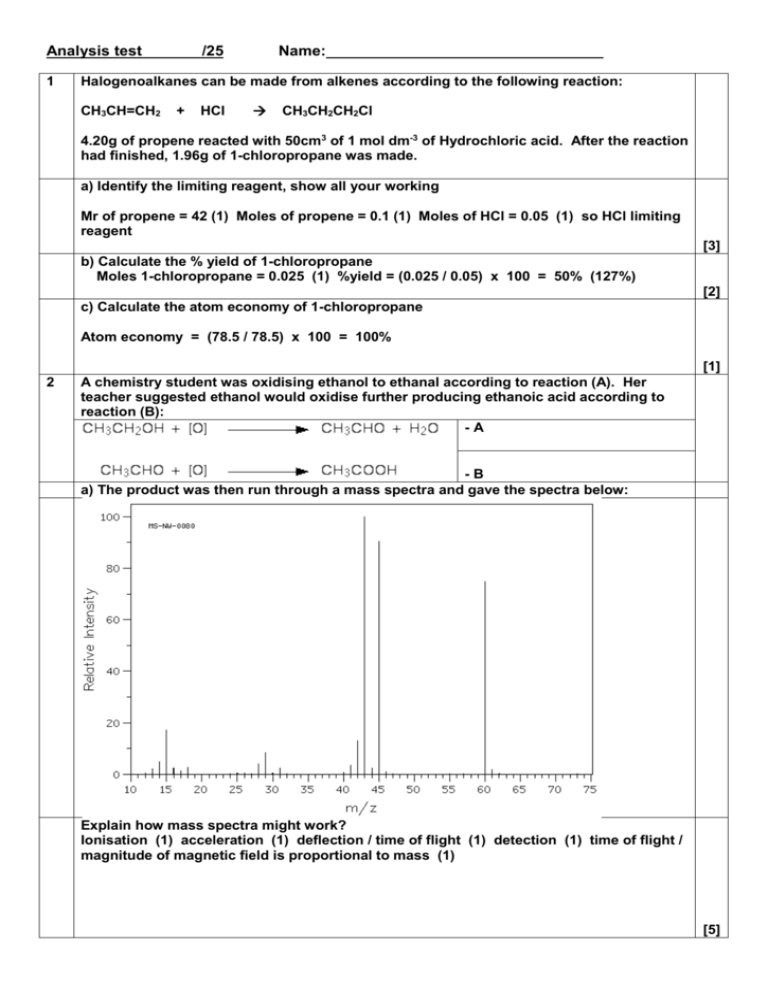
Analysis test 1 /25 Name: Halogenoalkanes can be made from alkenes according to the following reaction: CH3CH=CH2 + HCl CH3CH2CH2Cl 4.20g of propene reacted with 50cm3 of 1 mol dm-3 of Hydrochloric acid. After the reaction had finished, 1.96g of 1-chloropropane was made. a) Identify the limiting reagent, show all your working Mr of propene = 42 (1) Moles of propene = 0.1 (1) Moles of HCl = 0.05 (1) so HCl limiting reagent [3] b) Calculate the % yield of 1-chloropropane Moles 1-chloropropane = 0.025 (1) %yield = (0.025 / 0.05) x 100 = 50% (127%) [2] c) Calculate the atom economy of 1-chloropropane Atom economy = (78.5 / 78.5) x 100 = 100% [1] 2 A chemistry student was oxidising ethanol to ethanal according to reaction (A). Her teacher suggested ethanol would oxidise further producing ethanoic acid according to reaction (B): -A -B a) The product was then run through a mass spectra and gave the spectra below: Explain how mass spectra might work? Ionisation (1) acceleration (1) deflection / time of flight (1) detection (1) time of flight / magnitude of magnetic field is proportional to mass (1) [5] b) Identify the molecular ion peak m/z = 60 (1) [1] c) Who was right? The teacher of the student? Explain your answer Teacher (1) m/z = 60 which is Mr of ethanoic acid (1) if student was right would be m/z at 44 (1) [3] d) Write a chemical reaction to show how the molecular ion peak was formed CH3COOH + e- CH3COOH+ + 2e- CH3COOH+ (1) rest of equation (1) [2] e) Identify the fragment which is responsible for the peak at 15. Write a reaction to show how that fragment was formed. CH3+ (1) CH3COOH+ CH3+ + COOH Reactants (1) Products (1) [3] f) What other spectroscopic technique could used to distinguish between the 2 possible products? Infrared spectroscopy [1] g) Use the data sheet to predict what peaks would be present and absent in the 2 products Ethanal: C=O 1680 – 1750 (1) no O-H peak at 2500 – 3300 (1) Ethanoic acid: C=O 1680 – 1750 (1) Peak at O-H peak at 2500 – 3300 (1) [4]