File
advertisement

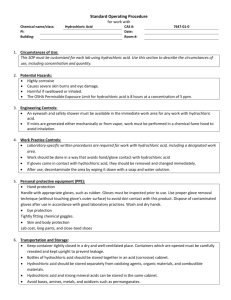
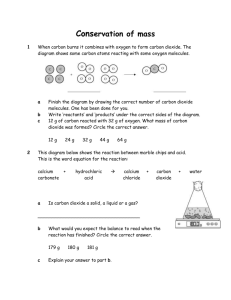
Lab Report Purpose To measure the effects of surface area on the reaction rate between a tums tablet and hydrochloric acid Background Information When a chemical reaction occurs the chemical bonds between the reactants change and products are formed. The reaction between the tums tablet and the hydrochloric acid is as follows: Calcium carbonate + hydrochloric acid Calcium chloride + water + carbon dioxide The rate of this reaction depends on how quickly the products are produced. For a reaction to occur two things have to be true: the activation energy must be achieved and then if this is the case, then a reaction will occur if a collision occurs between the reactants (calcium carbonate and hydrochloric acid). The investigation we did looked at the relationship between surface area and rate of reaction. Hypothesis The greater the surface area of the tums (independent variable) will mean there is a shorter time for the phenol red turns yellow (dependent). This is because when the tums tablet is crushed ie. Large surface area, there are more sides for the hydrochloric acid atoms to collide with the tums and therefore react and produce carbon dioxide. This carbon dioxide that is quickly produced is what turns the phenol red to yellow quickly. Materials - 30 ml Hydrochloric acid - 2 tums tablets - 20 drops of phenol red - Stopwatch - Mortar and pestle - 2 beakers Variables Independent – the surface area of the tums Dependent – the time it takes for the phenol red to turn yellow Controls – amount of hydrochloric acid, amount of phenol red, temperature, amount of tums Procedure 1. 2. 3. Data table Graph Conclusion The hypothesis was correct – it can be seen that the tums with the larger surface area reacted with the hydrochloric acid faster and the time taken for the phenol red to turn yellow was small. The crushed tums (large surface area) took 42 seconds and the whole tums (small surface area) took 4 minutes and 30 seconds. These results are due to the fact when a reaction occurs a collision between the reactants must occur and when there is a large surface area there is a greater area for these collisions to occur. Therefore there will be more collisions and more reactions so the products will be created much faster. Evaluation The experiment was fairly reliable as the results achieved were close to others in the class and was what was expected. One error could have come from the uncertainty of when exactly the phenol red had reached the correct shade of yellow for the stopwatch to be stopped. This could have been improved by using a yellow piece of paper that could have been used to reference the correct shade. Furthermore, when the tums was emptied from the mortar a small amount stayed on the edge. To improve this we could have used a scale to measure the mass of the tums to ensure it was constant.