The Chemistry of Natural Waters
advertisement

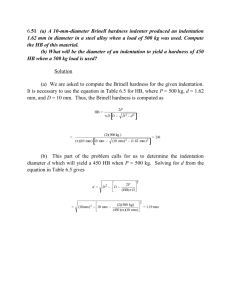
7 April 2015 Experiment 10 The Chemistry of Natural Waters CHEM 111 LAB REPORT ALEXANDRA EVERHART Basmah Alzayer Farouq Dajani Abby Ellington PENN STATE UNIVERSITY 2015 The Chemistry of Natural Waters Lab Report Introduction This lab was conducted in order to compare the hardness of water from different locations. Hardness of water refers to the number of minerals contained in the water. The main two minerals examined in the water are calcium and magnesium. Water is softened most often using a water softener, these water softeners take the magnesium and calcium out of the water by replacing it with sodium.(1) Water hardness can also be described as water’s ability to react with soap. The reason hard water does not react with soap very well is because soap tends to have a lot of anions, and magnesium and calcium (and other minerals) are positively charged (cations). This causes the cations to bond with the anions in the soap creating a thick greasy layer which is not effective for cleaning.(3) Being aware of water hardness is important because if there are too many minerals in the water it can affect the taste of the water. However, calcium and magnesium are necessary for human health and not having enough can also be detrimental. This is one reason why monitoring water hardness is important.(4) Also, hard water can leave precipitates in sinks around the drains and over long periods of time it can affect the quality of the pipes. Water hardness can be measured in parts per million, milligrams per liter, and grains per gallon.(3) Two common ways of measuring water hardness are the EDTA titration method and the AA method. The EDTA method tends to be slightly less accurate than the AA method. The AA Spectroscopy machine is more accurate because it uses more advanced technology and therefore it tends to give higher values of hardness than the EDTA titration. Figure 1 below is a schematic of how the machine works. Figure 1 In region one, the hollow cathode lamp on the left is the light source that emits a monochromatic light. This light is set for the specific element you are testing for. For this lab, two machines were used; one was set to Ca2+ and the other to Mg2+. The monochromatic light will be absorbed by whatever element energy level it is set for. The second region has a flame that at the same time sucks up the liquid water sample and turns it into an aerosol that can be broken into individual atoms. This way the atoms can absorb the corresponding wavelength of light hence the name atomic absorption. After this process, the light passes through the narrow slit that is set to only let the wavelength of Ca or Mg pass through it. Then, in the third region, the amplifier determines the intensity of the light, and the detector gives a value on the screen representing the amount of Ca or Mg in the sample. EDTA titration is a process of adding various chemicals to make a solution where its color will show the point where the Mg and Ca is depleted. The titration works by adding an NH3/NH4 buffer to a natural water sample. This buffer will bring the pH to 10, and then an indicator is added which turns the solution blue at a high pH. Next an EDTA solution is added to the titration. When the solution turns from blue to light purple, all the magnesium and calcium has reacted with the EDTA. If it does not turn light purple for a long time, this implies that the water is very hard because it has a lot of MG and Ca that needs to react with the EDTA. Two methods were also used to soften the water, one using baking soda and one using a cation exchange resin. EDTA titrations were done after each softening agent was added to determine the hardness. Our project was to compare the water hardness from different locations. This way we can compare the landscapes of each area with the quality of the water treatment plants in the area and see if this has an affect on the hardness of the water. There were four members in my group; Abby Ellington, Basmah Alzayer, Farouq Dajani, and myself. We each took a sample of water from a different location, my sample was tap water from my apartment in State College on South Allen Street, Basmah’s sample was taken in Miami, Florida, Farouq’s sample was taken in the North Hall dorms on Penn State’s campus, and Abby’s was from Amarillo, Texas. My hypothesis before was that both the samples taken in State College will have the highest levels of water hardness because State College is a karst landscape which means that it is “underlain by limestone that has been eroded by dissolution, producing ridges, towers, fissures, sinkholes, and other characteristics landforms.”(5) Miami’s geologic structure also contains limestone, but also bryzoan aspects which is sort of a mossy material.(6) This should still have some level of hardness because of the limestone but not as hard as the State College water because of the bryzoan aspects of the geology. Also, because it is near the coast there might be more of a saltwater intrusion problem than a water hardness problem. Abby’s sample from Amarillo, Texas comes from the Ogallala aquifer, and according to USGS the water tends to be hard.(5) This makes sense because limestone also underlies the Ogallala aquifer. From this data, I expect that Farouq’s and my samples will be the same in hardness, then Abby’s from Texas, and Basmah’s. Procedure Throughout this lab we completed four tests on the four different types of water: a TDS (total dissolved solids) test, an AA test, an EDTA (Ethylenediaminetetraacetic Acid) titration test, and two water softening method tests. For the TDS test, a drop of distilled water, our sample water, and a solution of 1x10-3 M Ca2+ were placed on a small piece of foil in a row next to each other.(3) Next, the foil was put on a hot plate to evaporate. After evaporation, it was noted what remnants or sediments were left. The amount of excess remains indicated how many minerals or how hard the water was.(3) After this, an EDTA titration was performed to see the point in the water when excess magnesium and calcium were present. We used a 1x12 well tray and put one drop of the ethylenediaminetetraacetic acid (EDTA) in well one of the tray, two drops in well two, three drops in well, three, four drops in well four, and continued this process all the way until well 12. One drop of buffer solution, one drop of EBT indicator which changes the color of the solution when there is a pH change or a reaction with Mg2+ and one drop of our water sample were added to each well.(3) Once this was done, it was noted at what specific well the color of the solution turned blue instead of purple. The blue indicated when there was excess EDTA meaning there was no longer enough magnesium and calcium to react with the EBT. My water was so hard that none of the first 12 wells turned blue, so I had to dilute it in a 1:1 ratio. This was accounted for in my calculations. After this using the equation MedtaVedta = MsampleVsample the hardness of the water was able to be calculated in molarity and then ppm and grains per gallon which will be shown in the results section.(3) Next, we compared two different ways of softening water. First 20 mg of baking soda was added to the natural water sample. Then, we performed the steps in the EDTA titration explained above using our water sample with a ratio of 20 mg of baking soda to about 10 ml of sample water. In order to compare which method was best we added about a fourth of a teaspoon of cation exchange resin and did another EDTA titration. After calculating the hardness of the water from the three separate titrations, a more accurate method of measuring the hardness of the sample water was used. Two different methods were used to compare the accuracy as well as to have more data to base conclusions from. An Atomic Absorption Spectroscopy (AA) machine was used. The AA machine has a very thin straw and the first step is to put it into a beaker of distilled water to calibrate it. The machine sucks up the water and measures it for hardness. So next, we put our samples in a beaker and placed the thin straw in our water to be tested for hardness. One AA machine measured calcium levels and one measured magnesium levels. If our sample was above a certain accepted threshold number for the machines accuracy we had to dilute it. I diluted my sample 1:1 so 5 ml of my water sample to 5 ml of distilled water. Figure 2 below is a picture of an AA Machine.(3) Figure 2 (2) Next we had to calculate the equivalent concentration on CaCO3 because this is the hardness of the sample, the actual mass of Ca and Mg in the water is not the exact same as hardness.(3) Results The summarized results of the total dissolved solids are as displayed below in table 1. The distilled water left an empty spot meaning there is no precipitate left. For the 1x10-3 M Ca2+ solution, a white ring was left showing there was a precipitate. My water sample and all of the water samples in our group left a white heavy spot. This means there is more calcium and precipitates in my sample then even in the water with calcium added to it. My partners Abby, Farouq, and Basmah all had very similar results. Table 1 In the EDTA titration, Farouq and I had to dilute our solutions in a 1:1 ratio. At first we did a titration without any water softening products and all three of us got higher hardness concentrations before adding the softening agents. This shows that the water softening agents worked. As you can see in table 2 below the baking soda softened the water on average by about 11.4% and the resin softened the water samples on average by 63.4%. So, the resin was much more effective in reducing hardness than the baking soda. The baking soda reduced the harness by 20 ppm for me, while the resin reduced the hardness of my sample by 190 ppm. Table 2 Shown below are the calculations from my EDTA titration that show how you find in the hardness in molarity, ppm, and grains per gallon. Every partner was required to do this for their sample, but below are just example calculations from my own experiment. As you can see below, the volume is measured in drops from a pipette. The molarity of the EDTA was given as 2x10-4. Also, in order to find the ppm hardness after using the softening agents, I would plug in 11 drops verse 11.5 for the baking soda softening method, and 5 drops instead of 11.5 drops for the resin softening method. Then, the same steps would be flowed to find the total hardness for those. In step three when converting ppm to grains per gallon the 17.1 number seen is just a simple conversion factor. Finding Initial Molarity MEDTAVEDTA = MSampleVSample (2x10-4)(11.5 drops) = MSample (2 drops) MSample = 0.0012 MSample = 0.0012 x 2(because I diluted my sample) So Molarity of hardness in my sample from state college = 2.4x10-3 M Converting Molarity to ppm (2.4x10-4 moles of CaCO3/ 1 liter of solution)*(100 g CaCO3/1 mole CACO3)* (1000mgCaCO3/1 g CaCO3) = 240 mg CaCO3/1 liter of solution = 240 ppm Converting ppm to grains per gallon 240 ppm* (1 grain per gallon/17.1ppm) = 14.04 grains/gallon The Absorbance values are summarized in table 3 below. Three of the four samples were too hard to be accurately measured by the machine so we had to dilute them 1 to 1. The numbers in Table 3 below are exactly what the AA machine output was. In order to account for the dilution, the numbers in the columns that have 1 to 1 dilution have to be doubled. Table 3 Table 4 below shows the Atomic Absorption Spectroscopy data in easier terms to understand of ppm concentration in each of the samples and the total ppm hardness. The calculations for how this was done are shown below. The numbers used for the example are the numbers from my water sample. The second two sets of calculations seen below are how to get the equivalent concentration of ppm to the total ppm harness or also known as the ppm of CaCO3 in the water. AA Ca Absorbance = 0.1776 (value from table 3) Y=0.007x +0.009 (equation from figure 3) Plug 0.1776 in for Y. -- 0.1176=0.007x + 0.009 x = 24.09 ppm x 2 (because of 1:1 dilution) x = 48.17 ppm of Ca in water from State College Apartment AA Mg Absorbance = 0.1729 (value from table 3) Y = 0.0103x + 0.014 (equation from figure 4) Plug in 0.1729 in for Y. -0.1729=0.0103x + 0.014 x = 15.43 ppm x 2 (because of 1:1 dilution) x = 30.85 ppm of Mg in water from State College Apartment. 48.17 ppm of Ca2+ *((100g/mole of CaCO3)/(40g/mole of Ca2+)) = 120.45 ppm CaCO3 or 120.45 ppm hardness 30.85 ppm Mg2+ *((100g/mole of CaCO3)/(24.3g/mole of Mg2+)) = 126.97 ppm CaCO3 or 126.97 ppm hardness Total ppm hardness = 120.45 ppm + 126.97 ppm = 247.38 ppm hardness Table 4 Figure 3 and Figure 4 are calibration graphs displaying the relationship between the light absorbance and metal ion concentrations. In both graphs one can see that as more light is absorbed the concentration of Ca2+ or Mg2+ increases. The formulas produced from these graphs were used to calculate the ppm from the values given from the AA machine. Figure 3 Figure 4 Discussion For the first part of our lab in the Total Dissolved Solids test corresponds to the results in that the Ca solution used on the foil had a molarity of 1x10-3 M of Ca2+ and the molarity of my sample was higher than that molarity with a value of 2.4x10-3 M. So it makes sense that more precipitate was left on the foil from the natural water samples than from the given Ca2+solution. The results from the water softener titrations are also as expected. The cation exchange resin took a significant amount more minerals out of the water than the baking soda. This makes sense because the cation exchange resin takes the Mg2+ and Ca2+ cations and replaces it with other cations that do not make the water as hard. The results from this experiment were similar and different to what was hypothesized. In table four one can see that the water from Penn State’s campus is significantly harder than any of the other samples. It was surprising that the sample from Penn State dorms is so much harder than the sample taken a mile away on South Allen Street in State college. It was assumed in the hypothesis that the hardness in these two samples would be close because the geology is the same but it was not considered that there are two separate water systems supplying Penn State’s campus and State College. Penn State gets its water from two well fields north of campus(7) while state college receives water from the State College Borough Water Authority.(8) So, the most likely reason why there is such a stark difference between the hardness although both samples were taken in the same town is that the two drinking water plants have different standards in the amount of minerals that is pumped out to its clients. The State College Borough Water Authority must have a more effective way of softening the water than the Campus’s method of softening the water for its faculty and staff. The sample from Amarillo Texas came in between had a hardness level of 302.95 ppm which is in between the two values from State College of 470.80 and 247.38 ppm. This makes sense because the landscape in Amarillo Texas as mentioned in the introduction also has a karst landscape similar to State College so for Abby’s water sample to be in between Farouq and I’s sample makes sense. The water sample from Miami, Florida had the lowest hardness at a ppm of 74.89. This is most likely because as predicted that the limestone which contributes to a karst landscape is not the largest factor contributing to the make-up of the geology in Miami, Florida. Although, these results make sense that does not mean there was not some human error. Farouq or I might have messed up our titrations and this caused our ppm hardness values to be so far apart not necessarily the fact that the water comes from two different water treatment plants.(8) Titration can be hard with the materials we were using because there is no guarantee that every drop that comes out of the pipette is the exact same volume as the one before it. The AA machine is more accurate because it has higher advanced technology that is able to take into account through using light wavelengths every particle of Ca and Mg whereas the EDTA titration method was not as accurate in this experiment because it was done my humans. Conclusion In this lab on the Chemistry of Natural Waters our main findings were that the water from Penn State’s campus is harder than the water in the surrounding State College area. Also, that even if water samples are taken in different states like Texas and Pennsylvania the hardness values can still be similar because of the karst geologic make-up. It was also found that drinking water on the coast in Florida has significantly less minerals causing hardness than areas more inland like Texas and Pennsylvania. References “Hardness in Drinking Water.” Department of Health. Agency of Human Services. Vermont.gov. http://healthvermont.gov/enviro/water/hardness.aspx#neg 1) “Atomic-Absorption Spectroscopy.” Science Hypermedia. 21 August 1996. http://elchem.kaist.ac.kr/vt/chem-ed/spec/atomic/aa.htm 2) 3) Thompson, Stevens PSU, Chem-trek Small Scale Experiments for General Chemistry 20142015 Hayden - McNeil Publishing MI USA 2014. Experiment #10, Sections A-G. Pgs 10:14-22. “Hardness in Drinking Water.” Background document for development of WHO Guidelines for Drinking-water Quality. World Health Organization. WHO Press. 2011. http://www.who.int/water_sanitation_health/dwq/chemicals/hardness.pdf 4) ”What is Karst?”. Environmental Science Institute. The University of Texas at Austen. 1999. http://www.esi.utexas.edu/outreach/caves/karst.php 5) 6) "Notes on Florida Geology." Notes on Florida Geology. September. 1997. http://www2.fiu.edu/~whitmand/Courses/Fl_geo_notes.html “Source Water Assessment Summary for the Penn State University.” University Public Water System. October. 2003. http://www.elibrary.dep.state.pa.us/dsweb/Get/Document59511/RW4140095001%20Penn%20State%20Univ%20--%20Univ%20Park.pdf 7) 8) State College Borough Water Authority. Web. (SCBWA). http://www.scbwa.org/about.php 9) Chemistry 111 Lab Notebooks. Alexandra Everhart. Spring 2015. Experiment 10. Sections A-G. Pgs 36-43. Abigail Ellington. Spring 2015. Experiment 10. Sections A-G. Pgs 29-34. Farouq Dajani. Spring 2015. Experiment 10. Sections A-G. Pgs Unknown. Basmah Alzayer. Spring 2015. Experiment 10. Sections A-G. Pgs Unknown. Book Source. Chemistry; Atoms First. A. Julia Burdge; Jason Overby. Special Edition – Pennsylvania State University. 2012. 10)