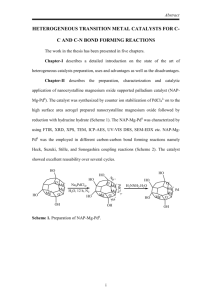
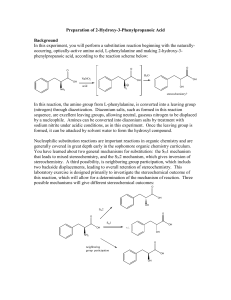
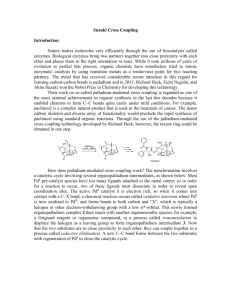
Suzuki Coupling The Suzuki Coupling is an organometallic reaction
advertisement

Suzuki Coupling The Suzuki Coupling is an organometallic reaction in which an aryl or vinyl borane is coupled to an aryl or vinyl halide through a palladium catalyst. It proceeds with retention of stereochemistry of the vinyl halide or bromide. In this experiment, you will conduct the reaction with a vinyl bromide of unknown stereochemistry. You will obtain the characterization for the final product, and use these data, with your knowledge of the mechanism, to determine the stereochemistry of the starting vinyl bromide. Procedure: Dissolve 3-methyoxyphenylboronic acid (84 mg), tetrakis(triphenylphosphine)palladium(0) (30 mg), and 1.1 mL of 1 M potassium hydroxide in 2 mL of THF in a roundbottom flask. Add 0.178 mL of 1-bromo-1-propene, attach a reflux condenser, and heat the reaction mixture to 60 oC in a water bath for one hour. Remove from heat. Add one mL of water to the crude reaction, then extract the reaction mixture with five 1 mL portions of pentane. Dry the combined pentane extracts with sodium sulfate, filter, and evaporate to obtain crude product. Filter the crude product through a 1 cm silica gel column with 5 1 mL portions of pentane to remove polar impurities. Collect the pentane fractions and evaporate to give purified product. Collect enough characterization data to determine the purity and identity of the product. (You will definitely need a proton NMR.) Questions to consider before lab: 1. What is silica gel chromatography? How does it work? (What you will do is actually more of a silica gel filtration.) Discussion questions: 1. Calculate your % yield. How successful was this experiment? The efficiency of the catalyst can be quantified in terms of Turn Over Number (TON). TON is defined as the number of catalytic cycles that occurred for each molecule of catalyst. Determine the TON for your reaction. (You will have to consider your % yield and the mol% of catalyst you used.) 2. Use your characterization data to discuss the identity and purity of the product. What was the stereochemistry of the starting vinyl bromide? References for a formal report. 1. Reference a Review paper to discuss the Suzuki Coupling in contrast to at least one other organometallic coupling reaction. 2. Reference a primary literature paper from the last year that uses the Suzuki Coupling. Why did they use it? How well did it work? Include a reactin scheme as appropriate.