Combined and Ideal Gas Law ProblemsKEY
advertisement
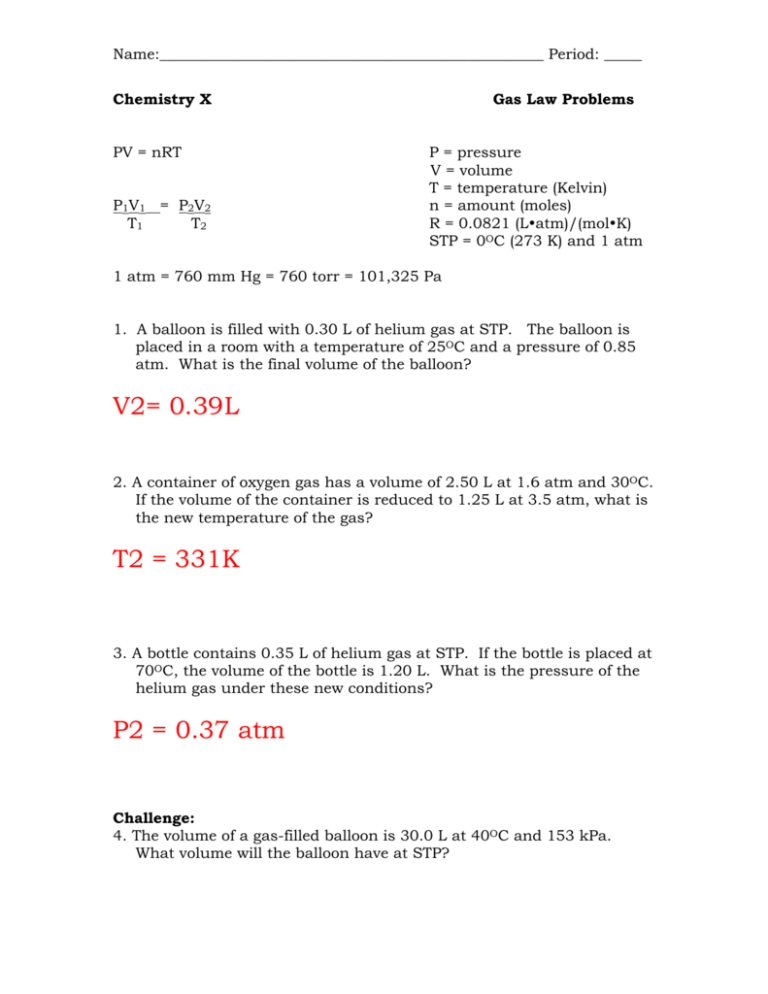
Name:___________________________________________________ Period: _____ Chemistry X PV = nRT P1V1 = P2V2 T1 T2 Gas Law Problems P = pressure V = volume T = temperature (Kelvin) n = amount (moles) R = 0.0821 (Latm)/(molK) STP = 0OC (273 K) and 1 atm 1 atm = 760 mm Hg = 760 torr = 101,325 Pa 1. A balloon is filled with 0.30 L of helium gas at STP. The balloon is placed in a room with a temperature of 25OC and a pressure of 0.85 atm. What is the final volume of the balloon? V2= 0.39L 2. A container of oxygen gas has a volume of 2.50 L at 1.6 atm and 30OC. If the volume of the container is reduced to 1.25 L at 3.5 atm, what is the new temperature of the gas? T2 = 331K 3. A bottle contains 0.35 L of helium gas at STP. If the bottle is placed at 70OC, the volume of the bottle is 1.20 L. What is the pressure of the helium gas under these new conditions? P2 = 0.37 atm Challenge: 4. The volume of a gas-filled balloon is 30.0 L at 40OC and 153 kPa. What volume will the balloon have at STP? V2 = 39.5 L 5. Given the following sets of information, calculate the unknown quantity. a. P = 1.2 atm; n = 0.1021 mol; T = 26.2 OC; V = ? V = 2.09 L b. V = 0.0275 L; n = 0.007812 mol; T = 16.6 OC; P = ? P = 6.75 atm c. P = 1.045 atm; V = 0.452 L; n = 0.002241 mol; T = ? T = 2546 K d. P = 0.6 atm; V = 0.0122 L; T = 298 K; n = ? n = 0.0003 mol 6. What volume does 4.24 g of nitrogen gas occupy at 58.2 OC and 2.04 atm? V = 4.07 L 7. At what temperature will 6.21 g of oxygen gas exert a pressure of 5.00 atm in a 10.0 L container? T = 3205 K 8. What pressure will be exerted by 0.450 mol of gas at 25OC if it is contained in a 0.650 L vessel? P = 16.9 atm 9. A rigid hollow sphere contains 685 L of helium gas at 621K. What is the temperature of the gas inside the sphere if it contains 251 moles of He (g)? P = 18.7 atm 10. You fill a rigid steel cylinder that has a volume of 20.0 L with nitrogen gas to a final pressure of 2.00 x 104 kPa at 28OC. How many moles of N2 (g) does the cylinder contain? n = 160 mol Challenge: 11. A deep underground cavern contains 2.24 x 106 L of methane gas at a pressure of 1.50 x 103 kPa and a temperature of 42OC. How many kilograms of CH4 does this natural-gas deposit contain? 2.1 x 10 4 kg CH4 12. A child has a lung capacity of 2.20 L. How many grams of air do her lungs hold at a pressure of 102 kPa and a normal body temperature of 37OC? Assume that the average molar mass of air is 29 g/mol. 250 g air 13. A 12.0g sample of oxygen gas is placed in a 1.76 L container at 25OC. What is the pressure of the gas inside the container? P = 5.2 atm










