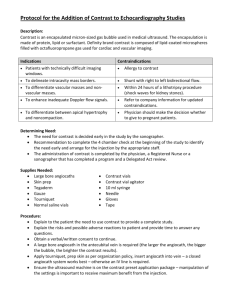
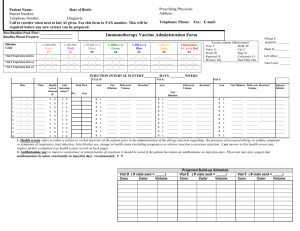
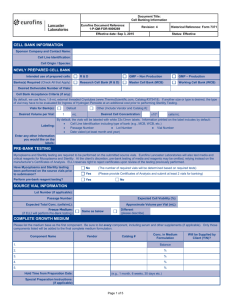
PURPOSE: 1.1 To lay down a procedure for preparation, storage
advertisement

1.0 PURPOSE: 1.1 2.0 SCOPE: 2.1 3.0 Assistant – QC ACCOUNTABILITY: 4.1 5.0 This procedure is applicable for all API, Intermediates and Raw materials in quality control department. RESPONSIBILITY: 3.1 4.0 To lay down a procedure for preparation, storage and usage of working standards. Executive – QC PROCEDURE: 5.1 5.2 Terminology: 5.1.1 Reference standard: It is the primary standard used for the generation of working standard. 5.1.2 Working standards: It is used for routine analysis. It is also be called as Secondary reference standard. 5.1.3 Reference sample: It is used for qualitative confirmations like Chromatography retention time, system suitability and IR spectrum identification. It is also called as Identification standard. These are prepared only when working standards are not available for the material. Selection of substance for working standard: 5.2.1 5.3 An approved batch of the substance shall be selected for its certification as working standard. Qualification of working standard: 5.3.1 The identified approved batch substance shall undergo analysis for Description, Identification, water / Loss on drying, Related substances and assay as per the respective Specification and Standard Testing Procedure (STP). 5.3.2 The analysis shall be performed using primary standards available from an officially recognized source or a well-established working standard, which is traceable to reference standard. 5.3.3 Where a primary reference standard is not available from an officially recognized source, reference standard received from R&D shall be used for evaluation. 5.3.4 Reference standard shall be certified by APL R&D. pertaining to its certification shall be retained. 5.3.5 The test for water shall be performed in duplicate and the test for assay shall be performed by two different weighing of reference standard and sample. The average value shall be reported. 5.3.6 The injection sequence is as follows Analytical data 5.3.6.1 System suitability solution - one injection. 5.3.6.2 Diluent - one injection. 5.3.6.3 Reference standard preparation 1 – Five injections. 5.3.6.4 Sample preparation 1 – Two injections. 5.3.6.5 Sample preparation 2 – Two injections. 5.3.6.6 Reference standard preparation 2 – Two injections. 5.3.6.7 System suitability solution - one injection. 5.3.6.8 Calculate the results as follows Assay 1: Sample preparation 1 Vs Reference standard preparation 1. Assay 2: Sample preparation 2 Vs Reference standard preparation 1. Assay 3: Sample preparation 1 Vs Reference standard preparation 2. Assay 4: Sample preparation 2 Vs Reference standard preparation 2. 5.3.7 The variation between the four assay results shall not be more than 0.5% w/w or 5µg/mg and it shall not be more than 1.0% or 10µg/mg with respect to the initial release result. 5.3.8 All related analytical data shall be recorded in the working standard protocol as per the Format No.: XXXXX-F01. 5.3.9 The working standard number shall be assigned as WS-XXX. Where : WS stands for working standard, and XXX is a continuous numeric serial number with respect to substance. 5.3.10 Enter details in the Working standard profile as per the Format No.: XXXXX-F04. 5.4 Storage and distribution: 5.4.1 The working standard shall be distributed into 16 amber coloured vials. 5.4.2 Working standard vials shall be labeled as per the Format No.: XXXXXF02. 5.5 5.4.3 The labeled vials shall be stored in a month wise plastic containers and stored in refrigerator. 5.4.4 Validity period for working standards shall be one year from the effective date and the effective date shall be with in one week from the date of completion of analysis. Usage of working standard: 5.5.1 As per the month, Month wise plastic containers containing working standards of different substances shall be issued for use. Vial under use shall be kept in desiccator, placed in the refrigerator. 5.5.2 The vial shall be discarded after one month from the date of issuance. 5.5.3 A fresh vial shall be issued after the vial in use is exhausted or after completion of one month from the date of issue. 5.5.4 List of working standards shall be maintained as per the Format No.: XXXXX-F07. 5.5.5 5.6 Working standard stock details shall be entered in the Format No.: XXXXX-F03. Reference sample: 5.6.1 An approved batch of the substance shall be selected for its certification as in house reference sample. 5.6.2 The working standards that are meant for qualitative analysis like identification and for system suitability test, only COA of that material shall be attached with the Reference sample certificate. 5.6.3 The Reference sample number shall be assigned as XXX. Where : XXX is a continuous numeric serial number with respect to substance. 5.6.4 Four vials shall be prepared and stored in the refrigerator. 5.6.5 Reference sample vials shall be labeled as per the Format No.: XXXXXF06. 5.6.6 Enter details in the reference sample profile as per the Format No.: XXXXX-F05. 5.6.7 Validity period for reference samples shall be two years from the effective date. 5.6.8 List of reference samples shall be mentioned as per the Format No.: XXXXX-F07. END OF DOCUMEN