Synthesis of Carboxylic Ester by reacting
advertisement

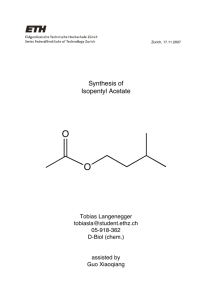
Synthesis of Carboxylic Ester by reacting Carboxylic acid and alcohol Chm 237 Monday @ 2:30 Khalid Alturaihy 4/23/13 Melvin R Simmons Abstract Applying Le Chȃtelier’ principle glacial acetic acid was esterified with Isopentyl alcohol to synthesize Isopentyl acetate. Reflux under water was used for synthesis. Distillation using a Hickman-stillhead was then used to distill the acetate. (.1grams, 8% yield). The melting point of the product was found (142˚ C), I.R. spectrometry was also used to determine the identity and purity of the product. The finished reaction presented a pleasant smelling compound comparing the melting point and the I.R. spectrometry to that of the literature confirmed the product to be Isopentyl acetate. [2] Simmons Introduction Esters are compounds that release vapors that are pleasant to smell such that similar to various fruits. They are using in a variety of things such as scented candles, air fresheners, incense and other sweet smelling products. The process for making air fresheners uses Fischer Esterification, the same procedure used to create our ester (Banana oil). Creating an artificial ester involves using various molecules containing alcohol groups (OH) and carboxylic acid groups (C=OOH), using different combinations will create different pleasurable scents. The chemical reaction is an equilibrium reaction, meaning that at the reactions termination there are often non-reacted reactants remaining in the solution at a ratio of products to reactants. This basically means that if the two chemicals were simply left to react with one another, many of the reactants would not react. Therefore, a few methods to push the reaction toward the product side to obtain a higher yield were used. Sulfuric acid (H2SO4) is used as a catalyst in the reaction. Large amounts of excess acetic acid will be present during the reaction in order to forcibly react more of the limiting reagent, in this case Isopentanol. As the reaction proceeds and the low boiling alcohol reactants will begin to leave the solution, the reflux apparatus is used to condense the alcohols and return them to the solution. Replenishing the alcohol avoids causing the products to go to the original reactant for. Once the reaction is complete, the ester product will be separated from the water product along with any remaining non-reacted reactants. Sodium bicarbonate is used to remove the acetic acid and sulfuric acid, and then distillation is used to remove any remaining water resulting in a pure ester liquid. [3] Simmons Determining the purity and identity of the product (hopefully an ester specifically Isopentyl acetate) left behind a boiling point and an I.R. spectrum are obtained. The smell of the Ester will also be a helpful indicator in determining if the correct compound was formed. Procedure A mixture of Isopentyl alcohol (.85grams 1.0mL) and glacial acetic acid (1.5mL) and 3 drops of sulfuric acid along with a spin vane, was added to a 5mL conical vial. The conical vial then assembled with the reflux apparatus and heated (150˚C) for 60-75 minutes. Once completed and cooled to room temperature, 1mL of sodium bicarbonate (NaHCO3) was added. When the reaction ceased the production of bubbles and gasses, the compound was allowed to separate; the lower aqueous layer was transferred to a flask and discarded as waste. This separation technique was done 2 more times with new 1mL sodium bicarbonate added each time. Finally, a microspatula-full of anhydrous sodium sulfate granules (Na2SO4) was added to the solution and the granules were left to absorb the remaining water impurities for 10-15 minutes. The distillation apparatus was then assembled with a thermometer positioned to measure the temperatures of the vapor from the distillation process will read on the thermometer. The dry esters were transferred to a 3mL conical vial with a spin vane and distilled. The distilled product was obtained and the weight and boiling point were recorded, finally the I.R. spectrum was obtained. [4] Simmons Results/Calculations Mass of starting alcohol: 23.99g-24.85g=.86 grams Mass of ending alcohol: 20.45g-20.55g= .10 grams* Product boiling point: 142˚C Smell: similar to that of a banana .86 𝑔𝑟𝑎𝑚𝑠 Moles of Isopentyl alcohol (limiting reagent) 88.148 𝑔/𝑚𝑜𝑙 = .00975 𝑚𝑜𝑙 .876 𝑔𝑟𝑎𝑚𝑠 Moles of acetic acid (excess reagent) 60.05 𝑔/𝑚𝑜𝑙 = .0145 𝑚𝑜𝑙 Theoretical yield was .86 grams (limiting reagent) Actual yield was .1 grams .1 𝑔𝑟𝑎𝑚𝑠 Percent yield .86 𝑔𝑟𝑎𝑚𝑠 𝑥100 = 11.76% yield Figure 1 located below shows the I.R. spectrum for Isopentyl acetate The I.R. spectrum shows that at just below 3,000 there is a C-H sp3 strong peak. It also shows the C=O around 1700 is a strong peak helping to identify the carbon to oxygen double bond. Figure 1 [5] Simmons Discussion/Conclusions At the end of the experiment, a successful esterification was performed from the starting acetic acid, using Isopentanol into the product of Isopentyl acetate. The reactants were heated using a reflux apparatus so none of the important products would be lost, helping serve as a catalyst in the reaction. Three extractions were performed to separate the organic later from the aqueous layer using 5% sodium bicarbonate. Any remaining water left over from the esterification process was dried using granular sodium sulfate, this allowed for a decently pure product. To further the purification process the product was brought to a boil using a HickmanStillhead distill; this technique is useful because Isopentyl acetate has a lower boiling point then the impurities left in the conical vial. The limiting reagent in the reaction is Isopentanol this was used to determine the theoretical yield. It could be determined by these calculations that the moles of ester should match that of the Isopentanol. When the ester was finally formed and weighed out it was determined that only 11.76% was yielded (full calculations found in results and calculations section). The yield was lower than expected due to the challenging equilibrium reaction along with a major spill of our pure ester. Even with the techniques explained in the introduction the equilibrium constant of the reaction was low and many reactants might have never reacted. The other possible explanation and most likely explanation would be the infamous spill that took place. When a spill as large as that it is quite apparent why we received such a low yield. The distinct banana smell that was present in the final product lead us to the observation that a successful esterification had been performed. The distinct banana smell is only present in Isopentyl acetate, so this was a good sign. The boiling point that was found (142˚C) helped to further the observation due to the literature reading a boiling point of 142˚C. The I.R. spectrum [6] Simmons (figure 1) further more supported our observation showing that at just less than 3000 a carbon to hydrogen sp3 bond was present and very strong. Also at around 1700 there was another sharp peak proving that there was a carbon to oxygen double bond present. Given all of these indicators towards a successful esterification it was determined that this lab was a success even with the small amount of recovery that was obtained. Next time more caution will be added to our open containers and cords floating around in the work space. [7] Simmons Questions from Page 68 1. Another method, involving the right hand side of the equation, which will favor the formation of ester, is to remove the product while the reaction is taking place. 2. It is easier to remove excess acetic acid from the products then excess Isopentyl alcohol because of solubility; specifically the acetic acid is more polar so it dissolves in the aqueous layer as opposed to the organic layer. 3. Sodium Bicarbonate reacts with acetic acid to make sodium acetate and carbonic acid. All of the acetic acid should be transferred to the aqueous layer after separation. 4. Isopentanol is the Limiting Reagent. Acetic acid is used in excess and has approx .002 moles more. 5. .876g/ml*1mL=.876 grams 6. 1mL/130mol/g=.00768 moles 7. (.0876g/mL)/(130)x(1/88)x100=58.8% yield 8. Direct esterification of acetic acid with Isopentyl alcohol, by using an excess of the starting materials shifting to the right in favor of the product. The excess are removed using extraction with sodium bicarbonate and water. After the drying with anhydrous sodium sulfate the ester is purified using distillation. 9. There is a strong band at 1742 cm-1 due to ester carbonyl group. At 1244 cm-1 due to CO-C Ether link. 1366 cm-1 due to CH(CH3)2 group 10. See Next Page for mechanism for esterification of acetic acid with Isopentyl alcohol. [8] Simmons [9] Simmons