Copper oxide
advertisement
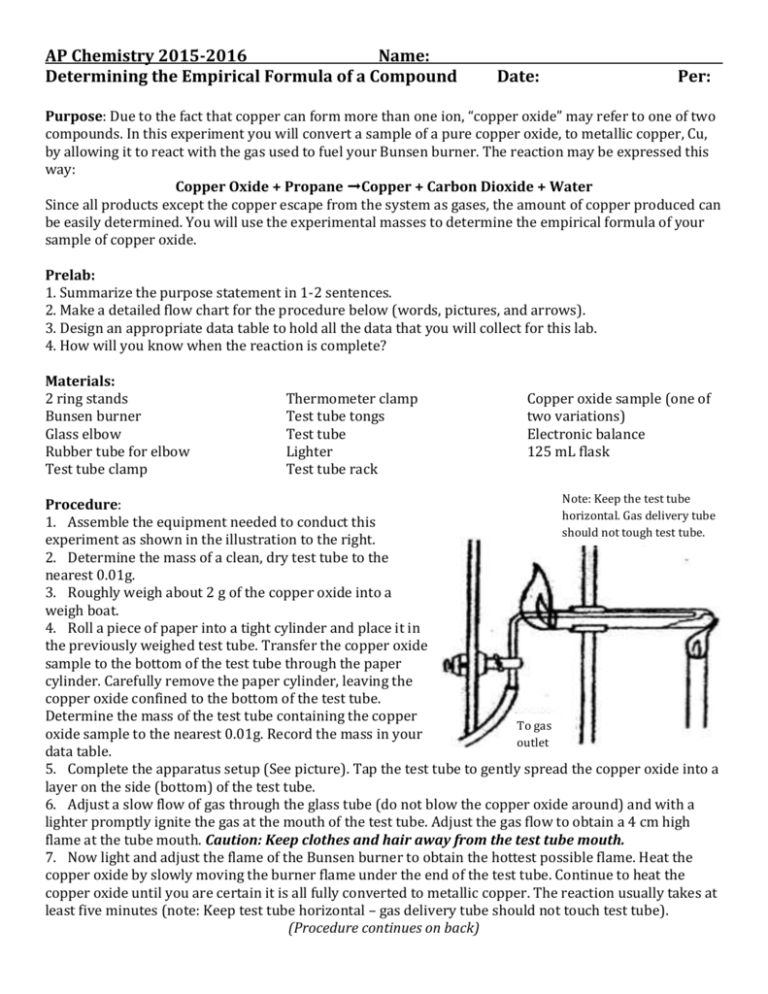
AP Chemistry 2015-2016 Name: Determining the Empirical Formula of a Compound Date: Per: Purpose: Due to the fact that copper can form more than one ion, “copper oxide” may refer to one of two compounds. In this experiment you will convert a sample of a pure copper oxide, to metallic copper, Cu, by allowing it to react with the gas used to fuel your Bunsen burner. The reaction may be expressed this way: Copper Oxide + Propane ➞Copper + Carbon Dioxide + Water Since all products except the copper escape from the system as gases, the amount of copper produced can be easily determined. You will use the experimental masses to determine the empirical formula of your sample of copper oxide. Prelab: 1. Summarize the purpose statement in 1-2 sentences. 2. Make a detailed flow chart for the procedure below (words, pictures, and arrows). 3. Design an appropriate data table to hold all the data that you will collect for this lab. 4. How will you know when the reaction is complete? Materials: 2 ring stands Bunsen burner Glass elbow Rubber tube for elbow Test tube clamp Thermometer clamp Test tube tongs Test tube Lighter Test tube rack Copper oxide sample (one of two variations) Electronic balance 125 mL flask Note: Keep the test tube Procedure: horizontal. Gas delivery tube 1. Assemble the equipment needed to conduct this should not tough test tube. experiment as shown in the illustration to the right. 2. Determine the mass of a clean, dry test tube to the nearest 0.01g. 3. Roughly weigh about 2 g of the copper oxide into a weigh boat. 4. Roll a piece of paper into a tight cylinder and place it in the previously weighed test tube. Transfer the copper oxide sample to the bottom of the test tube through the paper cylinder. Carefully remove the paper cylinder, leaving the copper oxide confined to the bottom of the test tube. Determine the mass of the test tube containing the copper To gas oxide sample to the nearest 0.01g. Record the mass in your outlet data table. 5. Complete the apparatus setup (See picture). Tap the test tube to gently spread the copper oxide into a layer on the side (bottom) of the test tube. 6. Adjust a slow flow of gas through the glass tube (do not blow the copper oxide around) and with a lighter promptly ignite the gas at the mouth of the test tube. Adjust the gas flow to obtain a 4 cm high flame at the tube mouth. Caution: Keep clothes and hair away from the test tube mouth. 7. Now light and adjust the flame of the Bunsen burner to obtain the hottest possible flame. Heat the copper oxide by slowly moving the burner flame under the end of the test tube. Continue to heat the copper oxide until you are certain it is all fully converted to metallic copper. The reaction usually takes at least five minutes (note: Keep test tube horizontal – gas delivery tube should not touch test tube). (Procedure continues on back) 8. Carefully heat the wall of the test tube to "chase out" any condensed moisture, which is another product of the reaction. 9. Remove the Bunsen burner, but allow the gas to continue to flow through the glass tube and to burn at the tube mouth. Wait 5 minutes or until the test tube is cool to the touch. When the tube has cooled turn off the gas flowing to the tube. There should no longer be a flame at the tube mouth. Extinguish it if necessary. 10. Carefully remove the cooled test tube from your equipment assembly and determine the mass of the tube with the metallic copper inside to the nearest 0.01g. Enter the results in your data table. 11. Shake the solid copper plug from the test tube and rub it firmly on a hard surface or hammer it. Can you observe the characteristic metallic luster of the copper? Is it malleable? Calculations: Calculate the empirical formula of the copper oxide. Show your work in detail. Analysis 1. What happened to the oxygen from copper oxide? 2. Write the balanced equation for the reaction of your copper oxide with propane (see purpose section). Conclusion Identify any potential sources of error in your experiment. Be specific! You may want to look up what the different forms of copper oxide look like to determine whether or not you were correct. Lab report due on Tuesday, September 22







