WS: U4 Review - Buckeye Valley
advertisement

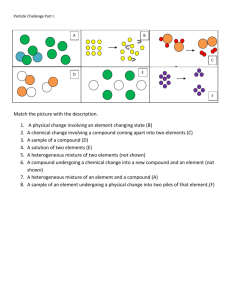
U4: Describing Matter: Review WS 2015 DUE 1/29/15 Must be completed by beginning of class on due date to be eligible for test retake! 1. I know the names and symbols of the assigned elements and the 7 diatomic elements. 2. I can distinguish between pure substance and mixtures. I can identify and sketch particle diagrams of compounds, elements, pure substances, mixtures. Sketch a particle diagram representing a mixture of hydrogen and oxygen gases. Sketch a particle diagram for the compound formed when these gases react. Describe how these diagrams are different. Label the following particle diagrams with ALL of the following terms that apply: compound, element, mixture, pure substance. Circle a molecule. Box an atom. 3. I can compare properties of a mixture to properties of the original substances and explain how differences in characteristic properties can be used to separate the components of a mixture. You are given a sample of a mixture composed of 50% sand, 40% sugar and 10% iron filings. Describe what steps you would take to separate the mixture into its components and the physical properties used in the separation process. Sketch a graph of temperature vs. time for the heating of a mixture of methanol and water. The b.p. of methanol is 65°C and that of water is 100°C. Describe how you could use the information in the graph you sketched above to separate a mixture of methanol and water. 4. I can state Avogadro’s Hypothesis and use this with evidence from combining volumes to deduce the formulas of some compounds. Avogadro’s Hypothesis states: Suppose that one volume of gas A combined with two volumes of gas B when measured at the same pressure and temperature. Sketch particle diagrams for molecules of gas A, gas B and the product; assume gases A and B are monatomic. Include a key to help distinguish the molecules. If the gases in the question above were diatomic, how many volumes of the gaseous product would be formed? Explain and draw particle diagrams to support your explanation. 4. I can state the Law of Definite Composition and draw particle diagrams to show it. I can state the Law of Multiple Proportions and draw particle diagrams to show it. 5. I state the 4 parts of Dalton’s Atomic Theory (Dalton’s model of the atom). a. b. c. d. 6. I can use masses to calculate percent composition of a compound. Two compounds of sulfur and oxygen are tested. Compound I contains 133.4 g of sulfur and 66.6 g of oxygen. Compound II contains 49.95 g of sulfur and 100.05 g of oxygen. a. Calculate the percent composition of sulfur and oxygen in each compound. b. Based on the percent composition by mass, would the two compounds have the same chemical formula? Why or why not? c. Explain how the example above demonstrates the Law of Definite Proportions. A 57.6 g sample of methane (CH4) is found to contain 43.2 g of carbon. a. calculate the percent by mass of carbon and hydrogen in the compound. b. How much hydrogen (in grams) would a 37.8 g sample of methane contain? 7. I can use a mass ratio data to predict the particle diagrams and write the formulae of compounds. Chromium and oxygen form several compounds. Two of these have the following mass composition: Compound A: 51.8 g of Cr and 22.2 g of O Compound B: 161.4 g of Cr and 102.0 g of O a. Determine the mass ratio of mass of oxygen in each compound. (e.g. 5.66 g A) mass of chromium A: __________ 1.00 g B B: ___________ b. How does the mass ratio for compound B compare to that in compound A? (assume the smallest ratio is 1:1) c. Sketch particle diagrams for the compounds of A and B that account for these mass ratios. Write the formula for the compound in each diagram. b. How does the example above demonstrate the Law of Multiple Proportions?