H2O - s3.amazonaws.com
advertisement

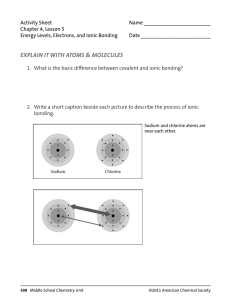
Chemical Bonds ~ Table Salt Salt, the most commonly known of which is sodium chloride, or table salt, is a compound formed by the chemical reaction of an acid with a base. During this reaction, the acid and base are neutralized producing salt, water and heat. Sodium chloride, is distributed throughout nature as deposits on land created by the evaporation of ancient seas and is also dissolved in the oceans. Salt is an important compound with many uses including food preservation, soap production, and de-icing roads and walkways. It is also the primary source of chlorine and sodium for industrial chemicals. In terms of chemistry, a salt can be any compound formed by the reaction of an acid with a base. Energy, in the form of heat, is given off during this neutralization reaction so it is said to be exothermic. The most common salt, sodium chloride (NaCl), is a product of the reaction between hydrochloric acid (HCl) and the base sodium hydroxide (NaOH). In this reaction, positively charged hydrogen ions (H+) from the acid are attracted to negatively charged hydroxyl ions (OH-) from the base. These ions combine and form water. After the water forms, the sodium and chlorine ions remain dissolved and the acid and base are said to be neutralized. Solid salt is formed when the water evaporates and the negatively charged chlorine ions combine with the positively charged sodium ions. Solid sodium chloride exists in the form of tiny, cube-shaped particles called crystals. These crystals are colorless, have a density of 2.165 g/cm3 and melt at 1,472°F (800.8°C). They also dissolve in water, separating into the component sodium and chlorine ions. This process known as ionization is important to many industrial chemical reactions Read more: Salt - Sodium, Water, Ions, and Reaction - JRank Articles http://science.jrank.org/pages/5948/Salt.html#ixzz3CCt0xaKa Chemical Relationships Chemical Equations of Water by Anthony Carpi, Ph.D. Chemical reactions happen all around us: when we light a match, start a car, eat dinner, or walk the dog. A chemical reaction is the process by which substances bond together (or break bonds) and, in doing so, either release or consume energy (see our Chemical Reactions module). A chemical equation is the shorthand that scientists use to describe a chemical reaction. Let's take the reaction of hydrogen with oxygen to form water as an example. If we had a container of hydrogen gas and burned this in the presence of oxygen, the two gases would react together, releasing energy, to form water. To write the chemical equation for this reaction, we would place the substances reacting (the reactants) on the left side of an equation with an arrow pointing to the substances being formed on the right side of the equation (the products). Given this information, one might guess that the equation for this reaction is written: H + O → H2O The plus sign on the left side of the equation means that hydrogen (H) and oxygen (O) are reacting. Unfortunately, there are two problems with this chemical equation. First, because atoms like to have full valence shells, single H or O atoms are rare. In nature, both hydrogen and oxygen are found as diatomic molecules, H2 and O2, respectively (in forming diatomic molecules the atoms share electrons and complete their valence shells). Hydrogen gas, therefore, consists of H2 molecules; oxygen gas consists of O2. Correcting our equation we get: H2 + O2 → H2O