Experiment 8 Basics of Membrane Transport
advertisement

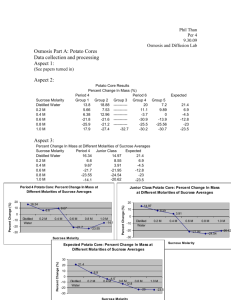
Mehmet Emre SUMER Koc University-Molecular Biology and Genetics 7 MAY 2010 MBGE 102- Experiment 8 Basics of Membrane Transport ABSTRACT: In this experiment our aim was seeing effects of different solutions on the red onion cells and calculating water potential of potato. To provide plasmolysis for onion cells the dilutions that have different concentration are dropped to red onion epidermis cells and the effects of dilutions are observed ender the microscope. Additionally, to determine the water potential of potatoes, small pieces of potatoes are put into plastic cubs that have different sucrose concentration and they are stood over night. After that, the mass of each group of potato cores is calculated. INTRODUCTION: Cell membranes is barier to enterance of most molecules, but not all. evolution of a cell membrane which can give permision some materials to pass while they are constraining the movement of other molecules was a major step in the evolution of the cell. Cell membranes are differentially permeable barriers separating the inner cellular environment from the outer cellular environment. Figure 1: Basic schema of membrane transports (1) Simple diffusion: Diffusion is the movement of molecules from a region of high concentration into a region of lower concentration. The difference in concentration between two regions is called the concentration gradient. Diffusion plays an essential role in the transport, over short distances of molecules such as nutrients, respiratory gases and neurotransmitters. Osmosis: Osmosis is the passage of water from a region of high water concentration through a semipermeable membrane to a region of low water concentration. A semi-permeable membrane is one that allows unrestricted passage of water, but not solute molecules or ions. Diffusion of water across a membrane - osmosis - creates a pressure called osmotic pressure. If the pressure in the compartment into which water is flowing is raised to the equivalent of the osmotic pressure, movement of water will stop. This pressure is often called water potential. Isotonic: The solutions being compared have equal concentration of solutes. Hypertonic: The solution with the higher concentration of solutes. Hypotonic: The solution with the lower concentration of solutes. Figure 2: Water relations in an animal cell (2) Figure 3: Water relations in a plant cell (2) Plasmolysis : If a cell is replaced in a hypertonic medium, the cell shrinks. Because the cell looses water. We call the shrinking of the cell as plasmolysis. Deplasmolysis: If a cell is replaced in a hypotonic medium, the cell swells. Because of the water transportation from outside of the cell to inside of the cell. We call this swelling as deplasmolysis. MATERIALS: - A red onion. A potato Bistoury Cover slips Plastic cubs Plastic wraps - Distilled water Micropipette Sucrose NaCl Balance Microscope METHODS: -Red onion plasmolysis: 1. We prepared a wet mount of the epidermis of a red onion with bistouries. 2. We added 2 or 3 drops of different NaCl solution to one edge of the cover slip. 3. Finally, we observed them under the microscope. -Calculating water potential with potato: 1. We prepared the following steps: -6 plastic cups containing 0, 0.2, 0.4, 0.6, 0.8 and 1 M sucrose solution -24 potato cores 2. We divided our potato cores into six groups of four. 3. We measured and recorded the mass of each of your six groups of potato cores. 4. We placed each group of potato cores in a different plastic cup. 5. We covered the plastic cups with plastic wrap. 6. We stood them over night. 7. The following day, we should have recorded the mass of each group of potato cores but we forgot to record them so they are rotted. RESULTS: Figure 4: The red onion cells shown here have been mounted in salty water. These cells are in a hypertonic solution and are therefore plasmoysis. Figure 5: The red onion cells shown here have been mounted in distilled water. These cells are in a hypotonic solution and are therefore deplasmoysis. DISCUSSION: REFERENCES: http://kentsimmons.uwinnipeg.ca/cm1504/membranefunction.htm(1) http://www.emc.maricopa.edu/faculty/farabee/BIOBK/BioBooktransp.html(2)