Chapter 16 Solutions
advertisement
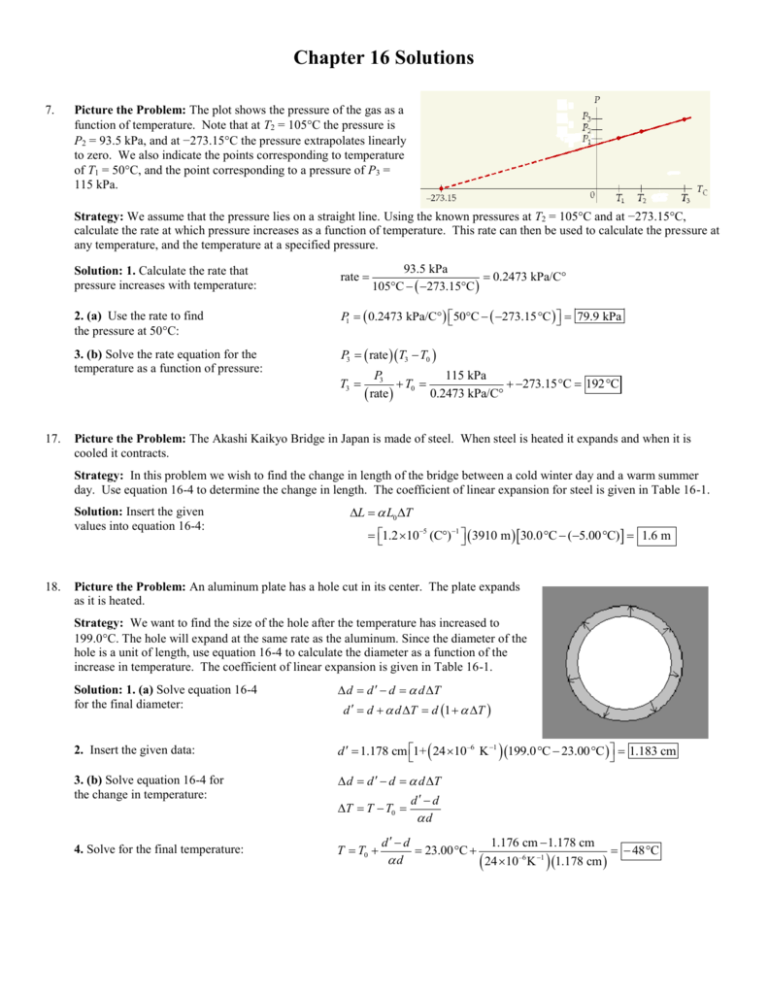
Chapter 16 Solutions 7. Picture the Problem: The plot shows the pressure of the gas as a function of temperature. Note that at T2 = 105C the pressure is P2 = 93.5 kPa, and at −273.15C the pressure extrapolates linearly to zero. We also indicate the points corresponding to temperature of T1 = 50C, and the point corresponding to a pressure of P3 = 115 kPa. Strategy: We assume that the pressure lies on a straight line. Using the known pressures at T2 = 105C and at −273.15C, calculate the rate at which pressure increases as a function of temperature. This rate can then be used to calculate the pressure at any temperature, and the temperature at a specified pressure. rate 2. (a) Use the rate to find the pressure at 50C: P1 0.2473 kPa/C° 50C 273.15 C 79.9 kPa 3. (b) Solve the rate equation for the temperature as a function of pressure: 17. 93.5 kPa 0.2473 kPa/C° 105°C 273.15°C Solution: 1. Calculate the rate that pressure increases with temperature: P3 rate T3 T0 T3 P3 115 kPa T0 273.15°C 192°C 0.2473 kPa/C° rate Picture the Problem: The Akashi Kaikyo Bridge in Japan is made of steel. When steel is heated it expands and when it is cooled it contracts. Strategy: In this problem we wish to find the change in length of the bridge between a cold winter day and a warm summer day. Use equation 16-4 to determine the change in length. The coefficient of linear expansion for steel is given in Table 16-1. Solution: Insert the given values into equation 16-4: 18. L L0 T 1.2 105 (C)1 3910 m 30.0 C (5.00 C) 1.6 m Picture the Problem: An aluminum plate has a hole cut in its center. The plate expands as it is heated. Strategy: We want to find the size of the hole after the temperature has increased to 199.0C. The hole will expand at the same rate as the aluminum. Since the diameter of the hole is a unit of length, use equation 16-4 to calculate the diameter as a function of the increase in temperature. The coefficient of linear expansion is given in Table 16-1. Solution: 1. (a) Solve equation 16-4 for the final diameter: 2. Insert the given data: d d d d T d d d T d 1 T d 1.178 cm 1+ 24 10–6 K 1 199.0 C 23.00 C 1.183 cm 3. (b) Solve equation 16-4 for the change in temperature: d d d d T d d T T T0 d 4. Solve for the final temperature: T T0 d d 1.176 cm 1.178 cm 23.00 C 48 C d 24 10–6 K 1 1.178 cm 21. Picture the Problem: A steel gasoline tank is completely filled with gasoline, such that the gasoline and the tank have the same initial volumes. When the gas and tank are heated, the gas expands more than the tank, causing some of the gas to spill out of the tank. Strategy: Since the initial volumes of the gas and tank are equal, the amount that will spill out is the difference in the increase in volume of the gas and tank, namely: The volume of spilled gasoline Vspill Vgas Vtank . Use equation 16-6 to calculate the changes in volume for the gas and tank. The coefficient of volume expansion for steel is 3 times the coefficient of linear expansion, which is given in Table 16-1. Solution: 1. Write the volume difference Vspill Vgas Vtank gasV0 3 tankV0 T gas 3 tank V0 T in terms of equation 16-6: 2. Insert the given data: 26. Vspill 9.5 104 3 1.2 105 C 0.93 L 3600 s 2.6 104 Cal/ s kg 75 kg 8.0 h 560 Cal h Picture the Problem: A lead bullet traveling at 250 m/s has kinetic energy. As the bullet encounters a fence post it slows to a stop, converting the kinetic energy to heat. Half of the energy heats the bullet resulting in an increase in bullet temperature. Strategy: Solve equation 16-13 for the change in temperature. Set the heat equal to one half of the initial kinetic energy of the bullet. The specific heat of lead is given in Table 16-2. Solution: Set Q in equation 16-13 equal to half the initial kinetic energy and solve for T: 38. 51 L 25 5.0 C Picture the Problem: The metabolic rate is the number of calories expended in bodily functions per second per kilogram. Strategy: We wish to calculate the total calories expended during a full night’s rest Multiply the metabolic rate by the person’s mass to calculate the calories expended per second. Multiply this result by 8.0 hours to calculate the calories expended in a full night’s sleep. Solution: Multiply together the metabolic rate, mass, and time: 36. 1 T 1 K Q 2 mc mc 1 2 1 2 mv 2 mc 250 m/s v2 120 K 4c 4 128 J/ kg K 2 Picture the Problem: Heat transfers from the hot lead ball to the cool water, causing the lead to cool and the water to heat up. Eventually the water and lead will come to the same equilibrium temperature. Strategy: Use equation 16-15 to calculate the equilibrium temperature. The specific heats of water and lead are given in Table 16-2. Solution: Insert the given data into equation 16-15: T mPb cPbTPb mw cw Tw mPb cPb mw cw 0.235 kg 128 J/ kg K 84.2 C 0.177 kg 4186 J/ kg K 21.5 C 0.235 kg 128 J/ kg K 0.177 kg 4186 J/ kg K T 23.9 C 41. Picture the Problem: A hot object is immersed in water in a calorimeter cup. Heat transfers from the hot object to the cold water and cup, causing the temperature of the object to decrease and the temperature of the water and cup to increase. Strategy: Since the heat only transfers between the water, cup, and object, we can use conservation of energy to calculate the heat given off by the object by summing the heats absorbed by the water and cup. Use the heat given off by the object and its change in temperature to calculate its specific heat. Solution: 1. Let Q 0 0 QOb Qw QAl and solve for QOb : QOb Qw QAl mw cw mAl cAl Tw 2. Solve for the specific heat using equation 16-13: cOb Qob m c mAl cA1 Tw T w w mOb T TOb mOb T TOb 0.103 kg 4186 J/ kg K 0.155 kg 900 J/ kg K 20 22 C 0.0380 kg 22.0 100 C cOb 385 J (kg C) 385 J (kg K) 3. Look up the specific heat in Table 16-2: The object is made of copper.