Biological agents
advertisement
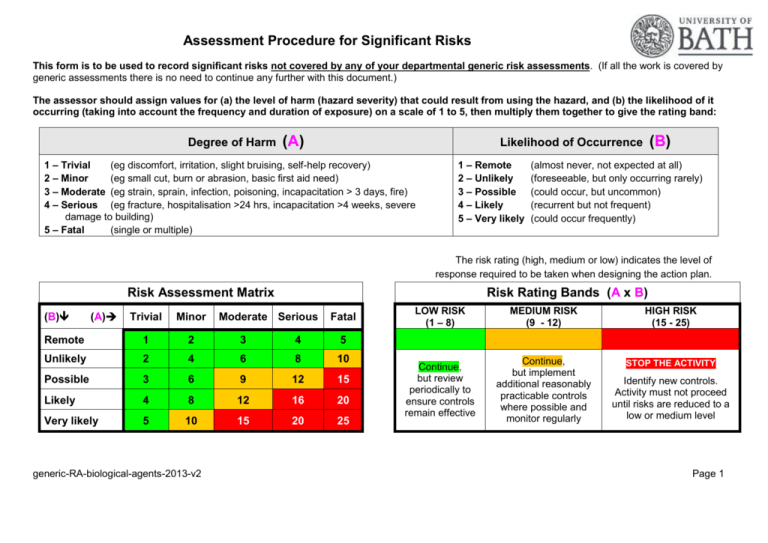
Assessment Procedure for Significant Risks This form is to be used to record significant risks not covered by any of your departmental generic risk assessments. (If all the work is covered by generic assessments there is no need to continue any further with this document.) The assessor should assign values for (a) the level of harm (hazard severity) that could result from using the hazard, and (b) the likelihood of it occurring (taking into account the frequency and duration of exposure) on a scale of 1 to 5, then multiply them together to give the rating band: Degree of Harm (A) (B) Likelihood of Occurrence 1 – Trivial (eg discomfort, irritation, slight bruising, self-help recovery) 2 – Minor (eg small cut, burn or abrasion, basic first aid need) 3 – Moderate (eg strain, sprain, infection, poisoning, incapacitation > 3 days, fire) 4 – Serious (eg fracture, hospitalisation >24 hrs, incapacitation >4 weeks, severe damage to building) 5 – Fatal (single or multiple) 1 – Remote 2 – Unlikely 3 – Possible 4 – Likely 5 – Very likely (almost never, not expected at all) (foreseeable, but only occurring rarely) (could occur, but uncommon) (recurrent but not frequent) (could occur frequently) The risk rating (high, medium or low) indicates the level of response required to be taken when designing the action plan. Risk Assessment Matrix (B) (A) Risk Rating Bands (A x B) Trivial Minor Remote 1 2 3 4 5 Unlikely 2 4 6 8 10 Possible 3 6 9 12 15 Likely 4 8 12 16 20 Very likely 5 10 15 20 25 generic-RA-biological-agents-2013-v2 Moderate Serious Fatal LOW RISK (1 – 8) MEDIUM RISK (9 - 12) HIGH RISK (15 - 25) Continue, but review periodically to ensure controls remain effective Continue, but implement additional reasonably practicable controls where possible and monitor regularly STOP THE ACTIVITY Identify new controls. Activity must not proceed until risks are reduced to a low or medium level Page 1 Generic Risk Assessment for biological agents Drafted by Pete Jewell Location of activity: July 2013 Policy and guidance for various hazardous biological agents is available at the UHS&E website (http://www.bath.ac.uk/hr/stayingsafewell/index.html) and the ‘1st Stop for Health & Safety’ wiki at https://wiki.bath.ac.uk/pages/viewpage.action?pageId=73829286. This risk assessment only covers work with hazardous biological agents. Any other significant hazards involved in the work activity must also be assessed in more detail using one of the more specialised generic, or specific, risk assessments available on the wiki. Once complete, all relevant workers must be informed, and their supervisor/manager must record their agreed ‘competence’ level (e.g. using a ‘supervision level’ of ‘low’, ‘medium’ or ‘high’) and get them to sign below. Date when will the activity is to start: Date when it is likely to finish: Assessor(s) names(s): Supervisor(s): Signatures of Date: Date of drafting assessment: Safety Coordinator: Date: Roughly how often will it be performed? Review date: Head of Department; Date: Supervision level. (Complete this after assessment is printed.) Supervisors and workers must jointly agree worker’s supervision level, defined as follows; 'LOW' - Workers are deemed to be adequately 'MEDIUM' - Activity cannot commence 'HIGH' - Activity cannot commence or proceed without direct senior supervision. trained and competent to perform this activity. without supervisor’s advice and approval. Worker’s name generic-RA-biological-agents-2013-v2 Worker’s signature Supervision level Supervisor’s name Supervisor’s signature Date Page 2 For help with the management of biological agents contact the relevant specialist via UHSE@lists.bath.ac.uk. . Specific risk assessments must be made for work; with prions or adenovirus with ACDP hazard group 3 or 4 agents for which HSE notification is required (some other specified pathogens cannot be used without Home Office authorisation). Refer to the ACDP Approved list. with any pathogen of plants or animals (or environmental samples which might contain them) for which authorisation from FERA is required with any genetically modified organism (GMO). Individual GM projects and personnel must be authorised by the University GM Safety Committee (contact gm@lists.bath.ac.uk) with infected animals (infected with pathogenic organisms including zoonoses) with toxins produced by biological agents (toxins are chemicals – refer to the generic risk assessment on the wiki) involving large scale culturing (>5 litres) of liquid cultures with Manduca moths, locusts, small mammals, nematode worms or wild birds (refer to Departmental Safety Coordinator) General Risk Control Measures. Completion of departmental safety induction is mandatory for lab workers. Lab coats must be worn by all workers in “wet” laboratories. Food or drink must not be consumed or stored in “wet” labs. Good standards of personal hygiene must be maintained at all times. Hand-toface operations must be avoided. Hands must be washed when leaving lab. Mouth pipetting or the use of mouth-operated suction devices is prohibited. POWDERED LATEX GLOVES ARE PROHIBITED IN THE UNIVERSITY. Lowprotein, non-powdered latex and nitrile disposable gloves (to EN374-2 and EN3743 Standard) should be available for use in laboratories. To avoid contaminating door handles and light switches lab gloves should not be worn outside labs. If necessary use one glove to carry lab items. All laboratories must maintain an inventory of any ‘dual-use’ materials to help with audits. This inventory must be checked at least annually. Spillages should be dealt with as soon as possible. If aerosols of hazardous agents may be present consider vacating the area to leave it to settle or dissipate before effecting decontamination. Decontaminate with appropriate liquids or powders (such as 70% alcohol, dilute hypochlorite (bleach) or Virkon), and clear up. Biological spillages may need disposal via autoclaving and if you are not sure how to dispose of any hazardous waste please contact your Technical Manager or email waste@lists.bath.ac.uk. If you are not sure SEEK ADVICE. First aid. For chemical spillages on body surfaces (exposed skin and eyes) the main aim must be to remove contaminating material as soon as possible. This is almost invariably achieved by flushing the affected area with cold tap water for up to 15 minutes. Each “wet” lab should have a hand-held emergency shower by each hand-wash basin adjacent to exit doors. (Additional advice on first aid treatment should be included in any specific risk assessment.) Where specific first aid treatment should be considered (e.g. when using cyanides or phenol) it must be detailed in the specific risk assessment involving those agents. For needlestick injuries, encourage the wound to bleed, rinse the wound site under cold water and follow any guidance given in specific risk assessments. Minor cuts can be treated locally. Basic first aid materials (adhesive wound dressings) should be readily available. For any injury at work, if irritation persists then you should seek medical attention. If your injury cannot be controlled then you should report to your GP, the University Medical Centre (200 metres to the west of the Building 2 South), the NHS Walk-in Centre in Bath or the Accident and Emergency unit at the Royal United Hospital in Bath. For emergency help the University internal telephone number 666 should be used. University Security staff are trained first aiders. A list of departmental or building first aiders should be displayed in departments. Incident reporting mechanisms. Injuries (even minor ones) dangerous incidents, and hazards should be reported on the A4 Incident report forms, available online at http://www.bath.ac.uk/hr/stayingsafewell/accidents-emergency/docsaccidentsandemergencies/Incident_report_form_V2.docx. generic-RA-biological-agents-2013-v2 Page 3 Particularly vulnerable groups at risk How could they be harmed? Risk before controls implemented A B Control measures needed to minimise risk A AxB Inexperienced workers (including undergraduate students, school pupils on “work experience” schemes and visitors). By skin/eye contact, inhalation, ingestion. 5 4 20 Supervisors must ensure all lab workers are provided with – a. Pertinent information. b. Competent instruction. c. Basic safety induction training. d. Relevant training for specialised tasks. e. Appropriate supervision. f. Suitable and sufficient risk assessments. Workers in a support role (technicians, lab assistants, porters) who need to clean surfaces or dispose of waste materials. By contacting contaminated surfaces or inhaling aerosols. 5 3 15 Ensure effective decontaminant solutions are available, workers know how to deal with spillages and that spills are cleaned up immediately. Pregnant women (and their unborn child). By being exposed to some specific biological agents capable of harming an unborn child. 5 3 15 Pregnant women must inform her supervisor or HR manager of her pregnancy. Supervisor must perform specific risk assessment. Persons with a history of particular medical conditions such as allergies to lab animals or latex, respiratory disorders, or those with skin conditions such as eczema, or those who have compromised or suppressed immunity through existing disease or medication By exposure to some agents capable of sensitising workers or exacerbating their condition. 5 3 15 If worker is aware of a particular sensitivity they must declare this to their supervisor or HR manager. Supervisors must identify work with known sensitisers, compile a risk assessment and request HR refer workers to the OH provider. Supervisor must perform specific risk assessment. Workers taking tissue (or blood) from themselves or lab colleagues. By reintroducing transformed cells into the donor (possibly via aerosol) it would not be recognised as ‘foreign’. 5 4 20 Such work is prohibited generic-RA-biological-agents-2013-v2 Risk after controls implemented B Page 4 AxB Hazard Who could be harmed? The propagation or culturing of biological agents, especially those that are pathogenic. Carrying out activities or processes which may produce aerosols of biological agents (including loading API strips and ELISA/microtitre plates). Handling sharp instruments such as hypodermic needles, scalpels, razor blades, broken glass or jagged metal which may be contaminated. Work with large quantities (e.g. > 5 litres) or high titres of biological agents. Workers and those in the vicinity Work with tissues from a high risk population or infected source or unscreened human or animal samples. Work with cell lines or strains that are not fully characterised, which are derived from high-risk sources or which may be contaminated with adventitious agents. The presence of unprotected wounds, abrasions or scratches on the hands, arms or face which may come into contact or be splashed with blood, blood-contaminated articles or pathogenic cultures. Worker generic-RA-biological-agents-2013-v2 How could they be harmed? Risk before controls implemented A A AxB Inhalation, ingestion, contact with mucous membranes. 5 3 15 Inhalation, than ingestion. 4 3 12 Puncturing the skin with contaminated item. 4 3 12 Larger volume work increases the risk. 4 3 12 Becoming infected with diseasecausing agent (such as bloodborne virus). 5 4 20 Becoming infected with diseasecausing agent. 5 3 15 Wound becoming infected with disease-causing agent. 4 4 16 Control measures needed to minimise risk Risk after controls implemented A B AxB All Any person working with genetically modified organisms (GMOs) must be authorised by the University GM Safety Committee (via the Biological Safety Officer) and follow relevant ‘Local Rules’ for GM work. GM projects must be similarly approved. Contact gm@lists.bath.ac.uk Avoid working with a viable pathogenic organism where possible or use the organism of least hazard consistent with the proposed work. Assign a hazard group (1 to 4 according to the ACDP criteria - refer to the ACDP Approved List) to infectious biological agents and only work in laboratories or rooms with the containment level and measures appropriate for that group. Ensure that appropriate storage, use, disposal and emergency procedures have been established before ordering any hazardous biological agent. Ensure you are using specified (and validated) decontaminant solution for microbiological spillages. Carry out manipulations or activities likely to generate an aerosol only in suitable microbiological safety cabinet (Class I for operator protection or Class II for operator and product protection, as defined by BS 5726:2005). Avoid the use of sharp objects or implements where possible. If unavoidable make sure that they are put into appropriate punctureresistant containers for safe disposal. Wear protective clothing (properly fastened laboratory coat and safety spectacles). Suitable and/or additional eye/face protection may also be necessary. Wear disposable gloves when handling infectious or contaminated material. Examine all personal protective clothing and equipment before use and replace any that are damaged or likely to be ineffective. Avoid hand to mouth/eye/face contact and thoroughly wash your hands before leaving the lab. Do not eat, drink, smoke, apply cosmetics or manipulate contact lenses in any “wet” laboratories or store-room where biological agents are kept or used. Page 5 Hazard Work with agents known to be drug-resistant or which have been genetically modified so that they are intentionally drug-resistant. Work with free plasmid DNA, particularly if it encodes oncogenic proteins and is designed for expression in mammalian cells. Who could be harmed? How could they be harmed? Worker, and people in the community Becoming infected with diseasecausing agent proving difficult to treat. Worker Potential for cancers to develop. Risk before controls implemented A A AxB 5 3 15 5 3 15 Possible carcinogenic effects caused by oncogenic viruses. 5 3 15 Physical damage caused by a biting or scratching animal. Workers and those in the vicinity Bites, possibly becoming infected. 5 3 15 Work with agents, especially parasites, which have variable life-cycle pathogenicity. Worker, and people in the community Contracting a disease caused by pathogenic stage of organism. 5 3 15 Toxic effects caused by microbial, animal or plant toxins. Worker Ingestion or skin/eye contact. 5 3 15 generic-RA-biological-agents-2013-v2 Control measures needed to minimise risk Risk after controls implemented A B AxB Do not mouth pipette or use mouth-operated suction devices to transfer culture media or any fluids. Decontaminate benches and other surfaces that may be contaminated at least once a day and whenever a spill of viable material has occurred. Keep freshly-made stocks of suitable disinfectant available within the laboratory. On a frequent basis, routinely replace the water in, and clean, water baths. This is also essential when a spillage of viable organisms or nutrient media has occurred. Place all infectious or contaminated microbiological waste in suitable containers for autoclaving or for soaking in decontaminant solutions. Human or animal wastes for incineration must be securely stored frozen in colour-coded plastic bags prior to despatch. Do not remove infectious or contaminated material from the laboratory to another area unless it is suitably packaged (double containment) in a sealed and labelled container. Disposable waste should be transported to the decontamination autoclave in closed bags inside the plastic holders designed for the purpose. Infectious materials may be centrifuged in the open laboratory if sealed rotors or sealed buckets are used and if these are opened only in a functioning microbiological safety cabinet. Immunisation and regular booster injections should be provided if appropriate as a supplementary safety precaution for those who may be exposed to pathogenic micro-organisms. Collect biological waste in suitable labelled containers for removal to the autoclave. Advice on any aspect of waste disposal can be sought from waste@lists.bath.ac.uk Page 6 Action Plan for additional control measures needed. Do any stated control measures need to be implemented before this activity can take place? Action Plan in respect of actions: Ref no. Prepared by: Action to be taken, including cost By whom Target date Responsible manager’s signature: Print name: Review date Outcome at review date Responsible manager’s signature: Date: Print name: Date: Assessment must be reviewed annually by responsible manager. generic-RA-biological-agents-2013-v2 Page 7