Supercritical Fluid Extraction Standard Operation Procedures
advertisement

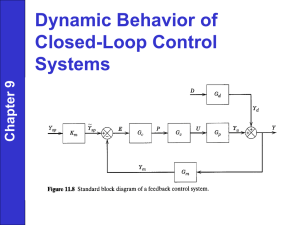
Supercritical Fluid Extraction (SFE) Standard Operation Procedures Introduction: Supercritical fluid is a substance that has been heated above its critical temperature. The critical temperature of a substance is defined to be the temperature in which it cannot exist as a liquid no matter what the pressure is. The critical pressure of a substance is the vapor pressure of the substance when it is heated to its critical temperature. The properties of a supercritical fluid fall in between the properties of the substance at its gaseous and its liquid phases. Supercritical fluids are particularly good at dissolving large nonvolatile molecules because of their densities. The supercritical fluid we will use in our extractions procedures is supercritical carbon dioxide. SFE is generally a relatively quick procedure. The rate of transfer between the sample matrix and the extraction depends on the rate of diffusion and the viscosity of the supercritical fluid. Most supercritical fluids are gasses at ambient temperatures allowing analyte recovery to be simple. Once the supercritical fluid is pushed through the extraction vessel, as the carbon dioxide equilibrates with the atmosphere, the analyte falls out of solution into the collection vessel. The SFE we will use consists of a liquid source of liquid carbon dioxide. There is a syringe pump that pushes the CO2 through the extraction vessel and through the outlet micrometer valve. This is where a collection vessel is placed in order to collect the analyte from the extraction procedure. The fluid then depressurizes and the analyte can be recovered in the collection vessel. Cleaning Procedure: 1. The recirculator needs to be turned on. This is the separate system on the far left that has hoses containing coolant running to and from it. The recirculator needs to run for a minimum of ten minutes before starting any experiments. 2. Check the Erlenmeyer flask labeled methanol. There should be enough methanol in the Erlenmeyer to have the head of the pump hose completely submerged. Replenish the methanol as necessary. 3. Turn on the cleaning pump (the unit on the immediate left of the SFE). The switch is located on the back side of the unit. 4. Check inlet, outlet and the vent valves to make sure they are all closed. Close them if necessary, then turn on the four switches on the front side of the SFE. You WILL hear an alarm sound, this is normal. 5. Take the sample vessel (the steel container with the ends that screw on) and open it. Place about quarter amount of glass wool in the vessel and pack it using the metal rod. 6. Place the vessel into the oven of the SFE and screw then valves into the proper ends of the vessel. If these are not tight, the vessel will leak and you will lose pressure. Do not forget to place the temperature probe against the vessel in the grooves. 7. Set the oven temperature to 100 C and the valve temperature to 110 C. 8. Turn on the CO2 tank (the valve directly on the tank first, then open the lines with the red handle) and use the knob on the front of the SFE to set the pressure to 240 bar. 9. Allow the oven and valve temperatures to reach their set point. Once they reach their temperatures, open the inlet valve and you will hear the SFE start to pump CO2. Allow the system to equilibrate back to 240 bar. Then open the outlet valve, and again, allow the system to equilibrate back to 240 bar. Once equilibrated, open the micrometer valve until you hear a steady stream of CO2 coming out. Adjust the valve as needed. 10. Purge the cleaning pump by pressing the purge button once, open up the purge valve (small white knob on the front of the cleaning pump) and then press purge again. When you see a steady flow of methanol coming out of the purge valve, close the valve and stop the purging process. Make sure the pump is set to 0.5 mL/min. 11. Turn on the cleaning pump and allow cleaning pump to run for 15 minutes to clean. After the cleaning process, turn off the cleaning pump. Experimental Procedure: 1. Follow the same procedures for the cleaning process, except the pressure will be set to 300 bar rather than 240. 2. You will then prepare the vessel by packing glass wool in one end, placing your weighed sample into the vessel, then packing the other end with glass wool. 3. Run the sample for 30 minutes, make sure to use a weighed Erlenmeyer (or beaker) with parafilm on the top to collect the sample. 4. When the sample is complete, close the outlet valve. (once the outlet valve is closed there is no need to do anything with the micrometer valve). 5. Close the inlet valve. Once closed, open the vent valve to release the stored pressure in the vessel. Once depressurized, remove the vessel from the oven (use gloves, it will be hot). 6. Open the vessel and the sample can be placed in the trash (for the wax extraction). 7. After running the samples, determine what the weight percent of wax in each sample is. Sample Preparation: Use about 1 g of sample When running the known sample, make sure the known percentage of wax is written down Shut Down: 1. 2. 3. 4. 5. Clean the SFE following the procedure outlined above. Once clean, close all the valves. Lessen the pressure to 50-60 bar using the knob. Use the vent valve to release pressurized CO2. Open the inlet valve to relieve internal pressure. Trouble Shooting: If the SFE is continually pumping while the system in equilibrating, open the oven to check for leaks. If there is a leak, make sure to relieve the pressure using the vent valve before trying to tighten the valves on the vessel. If the SFE is continually pumping and there are no leaks in the system, the CO2 tank may be running empty. Purpose: The SFE is used to isolate organic compounds from solid matrices using supercritical fluids. Carbon dioxide is readily available and generally used because it is non-toxic. We will be doing trial extractions of solo wax to determine the percent recovery of the SFE and then we will be extracting wax from wax paper samples to determine the wax content. Data: Solo Wax (g) 0.5251 0.4722 Erlenmeyer (g) 38.3237 39.3118 Sample: Wax Paper ID 1 2 3 4 5 6 2716 2716 2576 - 2579 2576 - 2579 2572 - 2575 2572 - 2575 Extracted Wax + Erlenmeyer (g) 38.8634 39.7542 Mass Wax Paper (g) 0.482 0.5137 0.4887 0.4684 0.4525 0.4438 Recovered Wax (g) 0.5397 0.4424 Average = Recovered Wax (g) 0.0872 0.1178 0.0662 0.1146 0.0551 0.077 % Recovery 102.780423 93.6891148 98.2347688 % Wax 18.09129 22.93167 13.54614 24.46627 12.1768 17.35016 Questions: Why does extraction efficiency of supercritical CO2 increase with density? Extraction efficiency of supercritical carbon dioxide increases with density because the solubility of the sample increases with the density of the carbon dioxide. What is the effect of temperature and why? Temperature affects the density of the carbon dioxide which in turn affects the solubility of it as well as its extraction efficiency. Conclusions: We were able to determine the extraction efficiency of the SFE to be 98.185%. We were able to extract wax from all of the unknown samples and the samples we ran were fairly consistent with the advertised values of the % wax. Overall the SFE lab was a success, although there were a few errors such as leaks and sample preparation.