IPS NAME Sections 4.1 - 4.4 Review Ws Date Period 1. Describe the
advertisement
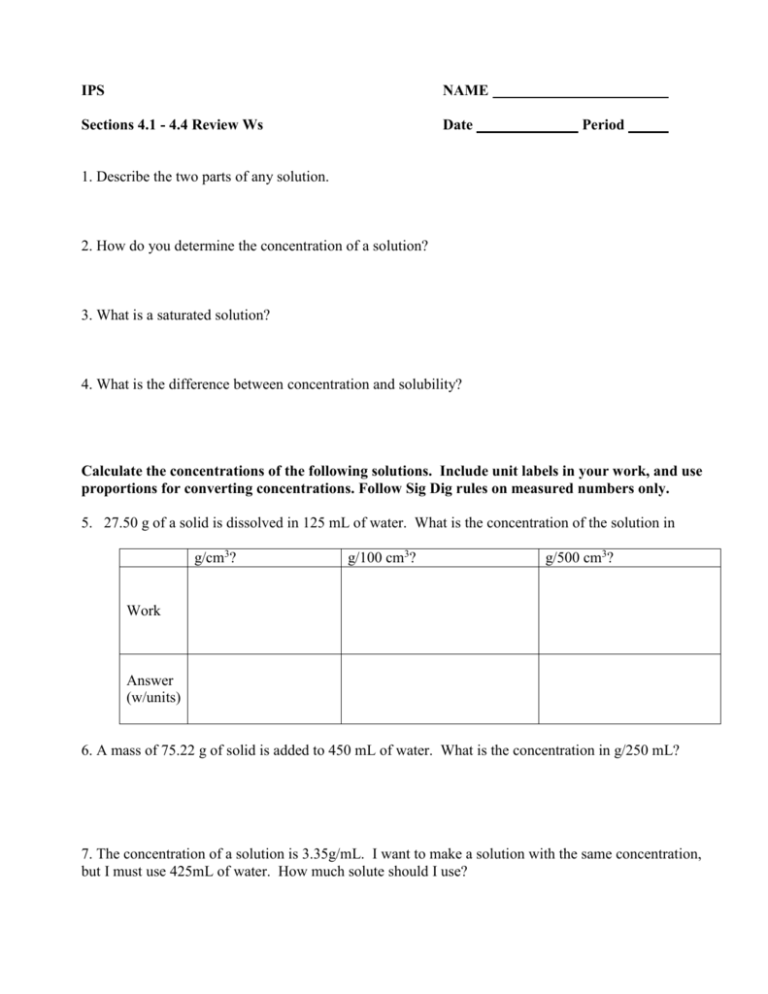
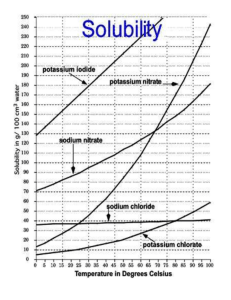
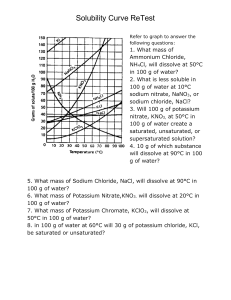
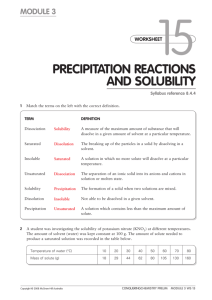
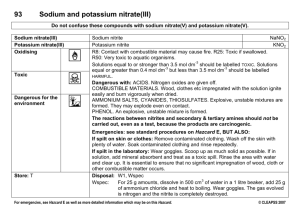
IPS NAME Sections 4.1 - 4.4 Review Ws Date Period 1. Describe the two parts of any solution. 2. How do you determine the concentration of a solution? 3. What is a saturated solution? 4. What is the difference between concentration and solubility? Calculate the concentrations of the following solutions. Include unit labels in your work, and use proportions for converting concentrations. Follow Sig Dig rules on measured numbers only. 5. 27.50 g of a solid is dissolved in 125 mL of water. What is the concentration of the solution in g/cm3? g/100 cm3? g/500 cm3? Work Answer (w/units) 6. A mass of 75.22 g of solid is added to 450 mL of water. What is the concentration in g/250 mL? 7. The concentration of a solution is 3.35g/mL. I want to make a solution with the same concentration, but I must use 425mL of water. How much solute should I use? 8. The concentration of a solution is 16.0 g/mL. I want to make a solution with the same concentration, but I must use 65 g of solute. How much water should I use? Use the Solubility graph to answer the following questions. 9. Which substance is most soluble at each if the temperatures listed below? a. 10ºC b. 65ºC c. 100ºC 10. Tell whether the following solutions are saturated or unsaturated. a) 100 g of sodium nitrate in 100 mL of water at 20 ºC. b) A solution of potassium nitrate in water with a concentration of 55g/100mL at 25 ºC? c) A solution of sodium chloride in water with a concentration of 30g/100mL at 70 º? 11. What is the solubility of Potassium nitrate at 75ºC? 12. At what temperature will 50g of Potassium Nitrate all dissolve? 13. a)How much Potassium Nitrate will dissolve in 100 mL of water at 85 ºC? b) How much will then dissolve in 30 mL of water at 85 ºC? 14. If I make a saturated solution of Sodium Nitrate at 20ºC, and then heat it to 70 ºC, how much solid needs to be added to keep the solution saturated? 15. A solution that has 110 g of Sodium Nitrate in 100 mL of water at 80 ºC is cooled. At what temperature will solid begin to precipitate? 16. When a saturated solution of Potassium Nitrate at 60 ºC is cooled to 20 ºC, How much solid was in the solution at 60 ºC? How much solid will stay dissolved ate 20 ºC? How much solid precipitates? 17. How does solubility of a solute change as it is heated? As it cooled?