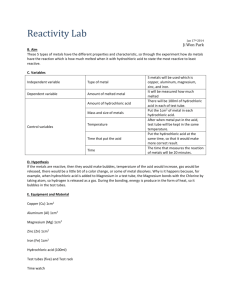
Reactivity of Metals Lab

Grade 11 Chemistry Name: _________________________________
Lab: Comparing Reaction Rates of Metals
Two single displacement reactions are studied in this lab:
M
(s)
+ H
2
O
(l)
M(OH) x(aq)
+ H
2(g)
and
M
(s)
+ HCl
(aq)
M(Cl) x(aq)
+ H
2(g)
Purpose: To compare the reactivity of various metals, to order them from least reactive to most reactive, and to discover the trend on the periodic table for reactivity of metals.
Materials: samples of Li
(s)
, Mg
(s)
, Ca
(s)
, K
(s)
, Na
(s)
, Cu
(s)
, Fe
(s)
, Zn
(s)
, 400 mL beaker, wire gauze, watch glass, tweezers, phenolphthalein indicator, water, dilute hydrochloric acid, 4 test tubes, test tube rack, tongs, sandpaper
Method:
Part 1: Testing the Reactivity of Alkali Metals and Alkali Earth Metals with Water
1) Fill the beaker half full with tap water. Add 2 drops of phenolphthalein indicator. Put the wire gauze over the beaker and have tongs ready for handling it if necessary.
2) Obtain a piece of magnesium from the front of the room on a watch glass. Gently sand the strip of magnesium. Describe several physical properties of the magnesium in the chart below.
3) While holding the wire gauze between you and the beaker (see the diagram) use the tweezers to drop the piece of magnesium into the water. Quickly replace the wire gauze and record your observations.
4) Dump the water and the products of the reaction into the sink. Carefully rinse the sides of the beaker
(occasionally metal will splatter onto these surfaces).
5) Repeat Steps 1 - 4 for Ca
(s)
, Na
(s)
, Li
(s)
and K
(s)
. Do not sand these metals, and handle them with tweezers only!
Part 2: Testing the Reactivity of Four Metals with Acid
1) Fill a test tube about ¼ full with dilute hydrochloric acid.
2) Obtain a piece of copper from the front of the room. Describe several physical properties of the copper in the chart below. Do not handle the metal!
3) Use the tweezers to place the metal into the test tube, and record your observations.
4) Place any excess metal in the garbage and all other chemicals down the sink.
5) Repeat Steps 1 – 4 for Cu
(s)
, Fe
(s)
and Zn
(s)
.
Observations:
Part 1: Testing the Reactivity of Alkali Metals and Alkali Earth Metals with Water
Metal
Li
(s)
Na
(s)
K
(s)
Mg
(s)
Ca
(s)
Physical Properties of the Metal
Part 1: Testing the Reactivity of Four Metals with Acid
Reaction with Water and indicator
Metal
Mg
(s)
Cu
(s)
Fe
(s)
Zn
(s)
Physical Properties of the Metal Reaction with Acid
Analysis: Answer the following questions on a separate piece of paper using full sentences.
1) List the elements in Part 1 from least reactive to most reactive in water.
2) List the elements in Part 2 from least reactive to most reactive in acid.
3) Metals will react more vigorously with acid than they will with water. With this knowledge, rank all the metals used in this lab from least reactive to most reactive.
4) State the trend for “reactivity of metals” across a period and down a group for the metals in Part 1.
5) Draw Bohr-Rutherford diagrams for the metals used in Part 1.
6) Give two reason why K
(s)
is more reactive than Na
(s)
. Then give two reasons why Na
(s)
is more reactive than Mg
(s)
. Refer to (i) the distance between the nucleus and the valence electrons and (ii) the number of shielding electrons in your answers.
7) Name an alkali metal that is more reactive than potassium.
8) Which atom do you think will react faster as it tries to obtain an octet of electrons in its outer orbit: F, Cl or O? Explain your choice.