Reactions - Fulton County Schools
advertisement

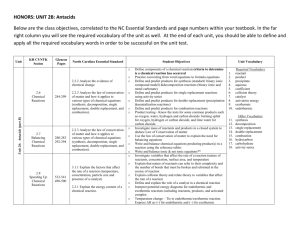
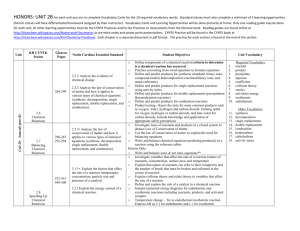
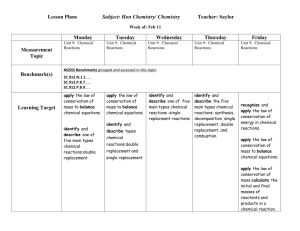
Department: _______Science_______________ Teacher: ___Davis___________________ Course: ____Honors Chemistry______________________ Topic / Theme: ____Chemical Reactions____________________________________________ ____ Essential Question: How can you predict the products of a chemical reaction and represent the reaction with a chemical equation? Key Questions: How do you write word equations, formula (skeleton) equations, balanced formula equations, and complete equations for a given reaction? How do you balance a chemical equation? How can you identify the five types of chemical reactions based on the reactants and products or reactants only? How can you predict the products of a single replacement reaction and write a correct equation given a periodic table and the reactivity series? How can you predict the products of decomposition reactions and write a correct equation? How can you predict the products of a combustion reaction and write a correct equation? How can you predict the products of synthesis reactions and write a correct equation? How can you predict the products of double replacement reactions and write a correct equation. What are the signs that a chemical reaction has occurred? How can you identify a reaction as exothermic or endothermic? Duration:___2.5 weeks________________________ Essential Vocabulary: Law of Conservation of Matter, Synthesis, Decomposition, Single Replacement, Double Replacement, Combustion, Precipitate, Chemical Equation, Coefficient, Complete Equation, Activity Series, Balanced Equation, Sentence Equation, Word Equation, Skeleton (Formula) Equation, Reactant, Product, Aqueous, Soluble, Insoluble, Catalyst, Complete Combustion, Incomplete Combustion, Exothermic, Endothermic Standards (List the primary standard and the key verbs from the elements) Higher-Order Student Engagement SC2 Students will relate how the Law of Conservation of Matter is used to determine chemical composition in compounds and chemical reactions. a. Identify and balance the following types of chemical equations: • Synthesis • Decomposition • Single Replacement • Double Replacement • Combustion b. Experimentally determine indicators of a chemical reaction specifically precipitation, gas evolution, water production, and changes in energy to the system. (Specific activities/tasks that will actively engage students in higher order learning) Assessment of Learning Goals : Formative and Summative Laboratories Call on specific students with content specific questions every day. Nightly Homework Problems. Unit Review Homework/Study Guide. Laboratory Activities. Unit Test (honors chemistry tasks require students to apply knowledge to solve problems) (How do you know if your students have learned?) Call on specific students with content specific questions every day. Nightly Homework Problems. Unit Review Homework/Study Guide. Laboratory Activities. Unit Test Activity Series of Metals Lab Classification, Balancing, and Observing Signs of Chemical Reactions Lab Conservation of Mass Lab (time permitting) We empower students to discover, think, and succeed. GPS Standards (Optional) SC2 Students will relate how the Law of Conservation of Matter is used to determine chemical composition in compounds and chemical reactions. a. Identify and balance the following types of chemical equations: • Synthesis • Decomposition • Single Replacement • Double Replacement • Combustion b. Experimentally determine indicators of a chemical reaction specifically precipitation, gas evolution, water production, and changes in energy to the system. We empower students to discover, think, and succeed.