LC2 - King`s Leadership Academy
advertisement
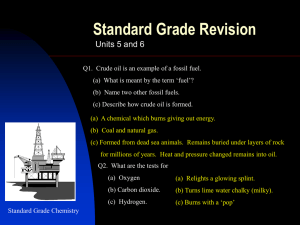
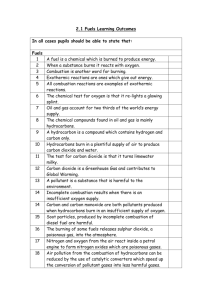
Year 10 Chemistry CORE Learning Cycle 2 Overview Is carbon the most important or most damaging element in the chemistry of life? Learning Cycle Overview: Line of enquiry 1: Hypothesis 1 Hypothesis 2 Hypothesis 3 Hypothesis 4 Hypothesis 5 Line of enquiry 2: Hypothesis 6 Hypothesis 7 Hypothesis 8 Is carbon the most important or most damaging element of life? Life is made of the same compounds as crude oil. Crude oil is used to fuel aeroplanes. Hydrocarbons all have similar properties as they are all made of the same things. Longer hydrocarbon chains release less energy than shorter chain hydrocarbons. The burning of fossil fuels is responsible for the damage of marine ecosystems. Week 1 Are polymers the most useful by-products of organic chemistry? Products of fractional distillation can be broken down further. Car tyres are made from plants. Cars can be run on corn. Week 2 Year 10 Chemistry CORE | Learning Cycle 2 | Medium Term Plan | Science 2015/16 Line of enquiry one: Is carbon the most important or most damaging element in the chemistry of life? Intentions for learning from AQA: Crude oil is a mixture of a very large number of compounds. A mixture consists of two or more elements or compounds not chemically combined together. The chemical properties of each substance in the mixture are unchanged. It is possible to separate the substances in a mixture by physical methods including distillation. Most of the compounds in crude oil consist of molecules made up of hydrogen and carbon atoms only (hydrocarbons). Most of these are saturated hydrocarbons called alkanes, which have the general formula CnH2n+2. Alkane molecules can be represented in the following forms: C2H6 Displayed formula. The many hydrocarbons in crude oil may be separated into fractions, each of which contains molecules with a similar number of carbon atoms, by evaporating the oil and allowing it to condense at a number of different temperatures. This process is fractional distillation. Some properties of hydrocarbons depend on the size of their molecules. These properties influence how hydrocarbons are used as fuels. The combustion of hydrocarbon fuels releases energy. During combustion the carbon and hydrogen in the fuels are oxidised. Most fuels, including coal, contain carbon and/or hydrogen and may also contain some sulfur. The gases released into the atmosphere when a fuel burns may include carbon dioxide, water (vapour), carbon monoxide, sulfur dioxide and oxides of nitrogen. Solid particles (particulates) may also be released. Sulfur dioxide and oxides of nitrogen cause acid rain, carbon dioxide causes global warming, and solid particles cause global dimming. Sulfur can be removed from fuels before they are burned, for example in vehicles. Sulfur dioxide can be removed from the waste gases after combustion, for example in power stations Lesson 1: Life is made of the same compounds as crude oil. Lesson 2: Crude oil is used to fuel aeroplanes. Key words: mixture, compounds, hydrocarbons, alkane. Key words: Boiling point, vapourised, saturated, properties. Learning Intentions: Students should develop an understanding that: Crude oil is made of a mixture of many compounds, called hydrocarbons, which are made of hydrogen and carbon. Hydrocarbons vary in chain length and can be named depending on the number of carbons in their chain. Hydrocarbons structures can be given using displayed and structural formula. Learning Intentions: Students should develop an understanding that: The many hydrocarbons which make up crude oil can be separated into fractions of similar chain length by fractional distillation. Each of the fractions of crude oil has a specific use. Success Criteria: Recall the elements that make up hydrocarbons. Suggest names for hydrocarbons. Model different hydrocarbon chain lengths using molymods and other modelling equipment. Feedback Focus: Knowledge input | Check | Development | REACH | Improvement Details: Peer assessment activity naming hydrocarbons. Teacher verbal formative assessment and use of rubric to keep record of feedback in books. Success Criteria: Identify the name of the process used to separate crude oil. Explain how factional distillation works. Research the uses of each chain length produced from fractional distillation. Feedback Focus: Knowledge input | Check | Development | REACH | Improvement Details: Self-assessed exam question using mark scheme. Home learning: Fractional distillation 6 Mark exam questions Year 10 Chemistry CORE | Learning Cycle 2 | Medium Term Plan | Science 2015/16 Lesson 3: Hydrocarbons all have similar properties as they are all made of the same things. Lesson 4: Longer hydrocarbon chains release less energy than shorter chain hydrocarbons. Lesson 5: The burning of fossil fuels is responsible for the damage to marine ecosystems. Key words: Cracking, unsaturated, catalyst. Key words: Combustion, particulates, incomplete, complete. Key words: Global warming, acid rain, global dimming. Learning Intentions: Students should develop an understanding that: Longer chain hydrocarbons can be broken down into smaller, more useful unsaturated hydrocarbons. Alkene double bond can be tested for using bromine water. Learning Intentions: Students should develop an understanding that: Longer hydrocarbon chains release more energy when they are combusted. Hydrocarbons produce water and carbon dioxide when combusted. Learning Intentions: Students should develop an understanding that: Combustion of hydrocarbons releases harmful products. These products have different environmental effects. Success Criteria: Build models for the first 4 alkenes. Explain the process of cracking. Describe how to test for alkenes. Feedback Focus: Knowledge input | Check | Development | REACH | Improvement Details: Assess redraft of 6 mark question from lesson 2 using rubric Teacher assessed of cracking equation questions Success Criteria: Recall products released by combustion of hydrocarbons. Propose hydrocarbon chain length for unknown molecules. Predict the properties of hydrocarbons based on their chain length. Evaluate investigation. Practical opportunity Comparison of the energy content of different fuels, for example by heating a fixed volume of water Success Criteria: Identify harmful products released by combustion of hydrocarbons. Explain the effects harmful emissions have on the planet. Research different methods used to limit harmful emissions. Decide which environmental effect is most damaging and justify your answer. Feedback Focus: Knowledge input | Check | Development | REACH | Improvement Feedback Focus: Knowledge input | Check | Development | REACH | Improvement Details: Self-assessment of investigation evaluation from previous lesson during starter. Teacher assessed 6 mark exam question on implications of burning fossil fuels Details: Peer assessment of analysis of results using mark schemes and graphs of answers. Homework: Research the different types of biofuels and their economic, environmental and ethical implications. Year 10 Chemistry CORE | Learning Cycle 2 | Medium Term Plan | Science 2015/16 Line of enquiry two: Are polymers the most useful by-products of organic chemistry? Intentions for learning from AQA GCSE specification:. Hydrocarbons can be cracked to produce smaller, more useful molecules. This process involves heating the hydrocarbons to vaporise them. The vapours are either passed over a hot catalyst or mixed with steam and heated to a very high temperature so that thermal decomposition reactions then occur. The products of cracking include alkanes and unsaturated hydrocarbons called alkenes. Alkenes have the general formula CnH2n. Unsaturated hydrocarbon molecules can be represented in the following forms: C3H6 Displayed formula Alkenes react with bromine water, turning it from orange to colourless. Some of the products of cracking are useful as fuels. Alkenes can be used to make polymers such as poly(ethene) and poly(propene). In these reactions, many small molecules (monomers) join together to form very large molecules (polymers). Polymers have many useful applications and new uses are being developed, for example: new packaging materials, waterproof coatings for fabrics, dental polymers, wound Lesson 6: Products of fractional distillation can be broken down further. Lesson 7: Car tyres are made from plants. Key words: Key words: Monomer, polymer, biodegradable. Learning Intentions: Students should develop an understanding that: The earth is made of three distinct layers: core, mantle, crust These layers are surrounded by the atmosphere Learning Intentions: Students should develop an understanding that: Alkene monomers are joined together to make large molecules called polymers. Polymers have an extensive range of uses including plastic bags. Most polymers are not biodegradable and cannot be broken down by microbes. Success Criteria: Describe the structure of the earth Describe the properties of each section including state of matter and relative temperatures Recall the outside layer is called the crust this is divided into sections called tectonic plates Practical opportunity – molymods/bromine water. Success Criteria: Propose names for polymers using alkene names. Use structure and bonding to explain properties of polymers. Write symbol equations for the polymerisation of ethene and propene. Feedback Focus: Knowledge input | Check | Development | REACH | Improvement Details: Redraft 6 marker based on teacher feedback Self-assessed task naming polymers and writing equations, Teacher verbal formative feedback with use of models. Year 10 Chemistry CORE | Learning Cycle 2 | Medium Term Plan | Science 2015/16 dressings, hydrogels, smart materials (including shape memory polymers). Many polymers are not biodegradable, so they are not broken down by microbes and this can lead to problems with waste disposal. Plastic bags are being made from polymers and cornstarch so that they break down more easily. Biodegradable plastics made from cornstarch have been developed. Biofuels, including biodiesel and ethanol, are produced from plant material. There are economic, ethical and environmental issues surrounding. Ethanol can be produced by hydration of ethene with steam in the presence of a catalyst. Ethanol can also be produced by fermentation with yeast, using renewable resources. This can be represented by: sugar carbon dioxide + ethanol Lesson 8: Cars can be run on corn. Key words: biofuels, hydration, catalyst, fermentation. Learning Intentions: Students should develop an understanding that: A range of alternative fuel resources are available using natural biological resources. Success Criteria: Recall the structural formula for ethanol. Describe the two methods for the production of ethanol. Evaluate which biofuels are best considering economic, ethical and environmental issues. Feedback Focus: Knowledge input | Check | Development | REACH | Improvement Details: Teacher assessment of home learning task through mini-test Peer assessment using mark scheme of exam question. Year 10 Chemistry CORE | Learning Cycle 2 | Medium Term Plan | Science 2015/16 Line of enquiry three: Are polymers the most useful by-products of organic chemistry? Intentions for learning from national KS3 science curriculum: In this line of enquiry pupils: