D6 Antibacterial
advertisement

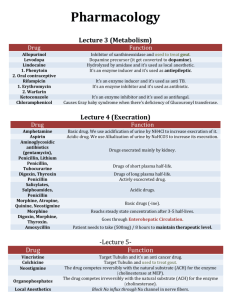
Review questions from 2003-2014 IB exams for option D Medicine and Drugs D1. Pharm 1. List 3 different ways in which drugs can be injected into the body. Predict (giving reason) which of the three will result in the drug having the most rapid effect. 2. What is meant by tolerance towards a drug and explain why is it potentially DANGEROUs? 3. State the meaning of the term parenteral 4. During drug development trials are conducted to determine the therapeutic window. Explain the meaning of the term therapeutic window and discuss its importance in drug administration 5. Explain the use of placebos in clinical trials in humans 6. Identify one other effect of a drug which must be determined during clinical trials 7. Drugs are most commonly taken orally. State one advantage and one disadvantage of this 8. List three methods, other than orally, that can be sued for administration of a drug. D2. Antacids 1. Antacid tables are used to neutralize some of the hydrochloric acid in the stomach. Two substances commonly used in the tablets are calcium carbonate and sodium hydrogencarbonate. Write an equation to represent each of these neutralization reactions 2. Explain with reference to the equations in #1 why a tablet containing .01 mol of calcium carbonate is more effective than one containing .01 moles of sodium hydrogencarbonate 3. Explain why alginates are often included in antacid tablets 4. Dimethicone is often included in antacid tablets because of its anti-foaming action. Explain with reference to #1 why dimethicone is included. 5. Identify another base used in antacid tablet for which dimethicone need not be included. 6. Name the acid found in gastric juice of the stomach 7. Explain why dimethicone is added to antacids 8. Sodium hydrogencarbonate (NaHCO3) and magnesium hydroxide ( Mg(OH)2 ) can both be used as antacids. Give the equation for the reaction of these two with hydrochloric acid 9. Compare the effectiveness of 1.00 grams of sodium hydrogencarbonate to .50 grams of magnesium hydroxide in combating acidity of the stomach 10. Explain why alginates are often added to antacids. 11. A well-known brand of antacids contains .160 grams of Al(OH)3 and .105 grams of Mg CO3 state the separate equations for the reactions of Al(OH) 3 and Mg CO3 with HCl aq 12. Determine which of the two components of the tablet with neutralize the most acid 13. The tablets also contain alginic acid and sodium hydrogencarbonate. The function of the sodium hydrogencarbonate is to react with the alginic acid to form sodium alinate. State the function of the sodium alginate produced. 14. On the leaflet which comes with the tablets it states that one of the side effects of the tablets is belching. Explain why this might occur. 15. Maalox manufactures several different types of antacids. Maalox extra strength is a suspension. One teaspoon (5.00 cm3) contains 400 mg of Mg(OH)2, 306 mg of Al(OH)3 and 40.0 mg of simethicone. Maalox extra strength with anti-gas comes in tablet form. Each tablet contains 1000mg of calcium carbonate CaCO3, and 60.0 mg of simethicone. a. State the equation for the reactions of magnesium hydroxide, aluminum hydroxide and calcium carbonate with hydrochloric acid b. Stomach acid approximates to .0100 M HCl. Assuming that simethicone does not react with the acid, determine the volume, in dm3 of stomach acid neutralized by -one teaspoon of Maalox extra strength (5.00 cm3) -one tablet of Maalox extra strength with anti-gas Suggest the function of the simethicone present in both antacids. D3. Analgesics 1. Explain the difference in the method of action of mild analgesic’s and strong analgesics 2. State the name of the nitrogen containing functional group in Paracetamol and heroin Paracetamol heroin 3. Naturally occurring morphine can be converted into synthetic heroin by reaction with ethanoic acid. Identify the group in the morphine molecule that reacts with ethanoic acid, the name of the type of reaction and the other product of the reaction A group in morphine molecule Type of reaction Other product of reaction 4. State the type of chemical reaction used to convert morphine into the semi-synthetic opiate heroin. 5. State the structure difference between the morphine and codeine molecules 6. State the main advantage and main disadvantage of using morphine as an analgesic 7. Outline two different types of social problems associated with the misuse of heroin. 8. Aspirin is thought to interfere with the production of prostaglandins. Explain how which produces an analgesic effect. 9. Explain how morphine can prevent pain 10. Paracetamol (acetaminophen) is generally considered to be safe to use as an analgesic in small doses. Other than the possibility of death, outline e problems associated with taking larger doses of Paracetamol. 11. State one important use of aspirin other than the relief of pain and fever 12. Other than the phenyl group state the name of one functional group that is common in both Paracetamol and morphine Both aspirin and diamorphine 13. Explain what is meant by the term tolerance and suggest why this is a particular problem for heroin users. 14. State the differences between structures of morphine and diamorphine (HEROIN) state the names of all functional groups in the molecule of morphine. 15. State one advantage of aspiring and one disadvantage of Paracetamol. 16. Explain why it is dangerous to take aspirin when ethanol has been consumed. 17. Discuss one advantage and one disadvantage of taking diamorphine rather than morphine to relieve pain 18. Discuss the different modes of action of paracetamol (acetaminophen) and codeine in relieving pain. 19. Diamorphine (heroin) is an even stronger painkiller than codeine. The structures of codeine and diamorphine are given in earlier questions. Discuss in terms of named functional groups, how the structure of diamorphine differs from the structure of codeine. 20. State one specific reason why doctors recommend paracetamol rather than aspirin as a mild analgesic. 21. A normal aspirin tablet taken to relieve pain contains about 300 mg of aspirin. Certain adults who are not in pain are recommended by doctors to take a smaller 75 mg dose of aspirin each day. State one reason for this recommendation. 22. Suggest a reason why aspirin and paracetamol are “over the counter drugs” but in many countries solpadol must be prescribed by a doctor. D4 Depressants 1. One method for detecting alcohol in breath involves blowing through a tube containing crystals of acidified potassium dichromate (VI). The alcohol turns the crystals from orange to green. Explain what happens to both the dichromate (VI) ion and the alcohol in this reaction. What species is responsible for this final color? 2. A modern method for accurately determining the amount of alcohol in breath uses an intoximeter. Explain how this works. 3. Suggest why it is advisable not to drink alcohol when taking other drugs. 4. Depressants such as tranquilizers and sedatives are capable of affecting the CNS (central nervous system). State two effects on the body by taking a low dose of tranquilizer and two effects by taking a high dose of a sedative. 5. Explain why depressants are sometimes describes as anti-depressants 6. The most widely used depressant is ethanol. Discuss the harmful effects of regularly taking large amounts of ethanol by referring to 4 specific problems 7. List 3 prescription depressants 8. One problem with many drugs is that users develop tolerance. Explain what is meant by the term tolerance and state why it could increase the risk to the user. 9. Explain how the breathalyzer works and describe its color change in a positive reaction 10. Describe one effect at a moderate dose and at a high dose of depressants on the human body that are medicines prescribed by doctors. 11. One method used to check whether a driver has consumed ethanol is the breathalyzer. This makes use of an oxidation reaction of ethanol. For this reaction, Identify the reagent used The color change A possible organic product 12. Fluoxetine hydrochloride (Prozac) is a common depressant. Depressants have many therapeutic uses. State three other common depressants and describe one effect other than relieving depression of moderate doses of depressants on patients. 13. Ethanol is a depressant that can be consumed in the form of alcoholic drinks. The danger is that there is little control over the amounts used. Discuss the long term consequences of ethanol abuse, both for the individual and for society. 14. Which type of depressant is more water soluble and why (according to its chemical structure) 15. Deduce the full balanced chemical equation for the redox reaction of ethanol with acidified potassium dichromate (VI) and state the name of the organic product formed 16. In order to quantify exactly how much ethanol is present in the blood, a person may be required to give a blood sample or may be asked to blow into an intoximeter. Explain the chemistry behind the techniques for determining the ethanol content in a blood sample and by using an intoximeter Blood: Intoximeter: 17. Ethanol may exert a synergistic effect when taken with other medicines. State the meaning of the term synergistic effect. D5 stimulants 1. 2. 3. 4. Methyl amphetamine On the structure of methamphetamine above draw a ring around the amine group. Determine whether both amine groups in caffeine are primary, secondary or tertiary Caffeine contains the group 0 CH3 state the general name for this functional group ---C------N---A designer drug with a structure related to methyl amphetamine is ecstasy. Ecstasy tablets are sometimes contained with a substance called 4-MTA. Ecstasy 4-MTA Methyl amphetamine, ecstasy and 4-MTA are sympathomimetic drugs. Identify the structural similarity between the three drugs and adrenaline, the structure of which is giving below Adrenaline Outline what is meant by the term sympathomimetic drug and state on example of the short term effect sympathomimetic drugs have on the human body. 6. State on example of a long term effect of taking stimulants 7. Describe two similarities in the structure of caffeine and nicotine (not including the presence of double bonds, methyl groups and nitrogen atoms) compare the structures of caffeine and nicotine in terms of functional groups 5. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. Discuss the problems associated with nicotine consumption, distinguishing between shortterm and long term effects. State the name of the class of drugs with effects similar to that of adrenaline. Outline one effect of these drugs on humans. Identify the stimulant responsible for the addiction to smoking tobacco List three physiological effects of stimulants Name the type of drug that increases mental alertness Explain the term sympathomimetic drug Identify 2 structural differences between amphetamine and adrenaline State the effect of caffeine on the urinary system Identify the functional group present in both caffeine and nicotine Describe two effects of large amounts of caffeine on the human body Deduce which functional group is common to both nicotine and caffeine. Draw the structure of phenylethylamine Compare the two nitrogen containing groups of amphetamine and epinephrine. Caffeine is found in chocolate along with another stimulant called theobromine. identify the Alkene group in theo bromine above by drawing a ring around it. 22. State the name of the functional group in the theobromine circled. 23. State the name and classification of the N-CH3 group in the 5 membered ring. 24. Caffeine contains both amide and amine functional group. Identify the amine group in caffeine by drawing a circle around it 25. Caffeine is a stimulant and also a diuretic. State the meaning of the term diuretic 26. Nicotine is also a stimulant. Both caffeine and nicotine contain a tertiary amine group. Distinguish between the terms primary and tertiary when applied to amines. 27. D6 Antibacterial Penicillins are molecules that can kill harmful micro-organisms. The general structure is shown 1. State the type of micro-organism killed by penicillin and explain how they do this 2. Explain the effect of over prescription of penicillin 3. Describe the differences between bacteria and viruses, by referring to their structures and the way they multiply. 4. Outline how penicillin work as antibacterial 5. Compare broad spectrum and narrow spectrum antibiotics 6. Discuss the problem with over prescription of penicillin. 7. Penicillin G in which the R group is C6H5CH2 deduce the number of carbon atoms in one molecule of penicillin G 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. Some bacteria are resistant to penicillin explain how these bacteria are able to resist the effect of penicillin G Describe how the structure of penicillin was modified to overcome this problem Explain how penicillin are able to destroy bacterial A doctor prescribes a broad spectrum antibiotic for a patient then some days later a narrow spectrum antibiotic. Describe what the doctor does not allow the medication to be changed to the narrow spectrum antibiotic State the main disadvantage of using a broad spectrum antibiotic. Outline the major contribution of Florey and chain in the development of penicillin Discuss the reason why a “cocktail” of different antibiotics may be needed to treat tuberculosis. Many diseases are the result of infection of the body by either bacteria or viruses. State the name of one disease caused by bacterial ______________ and viruses _____________ Describe two misuses of antibiotics that have led to some bacteria becoming resistant The discovery of penicillin by alexander Fleming in 1928 is often given as an example of serendipity in science. Describe ether chance event that led to alexander Fleming’s discovery of penicillin. Outline the work of Florey and chain in developing penicillin Explain how penicillin works and why it is necessary to continue to develop new forms of penicillin with modified side chains. Two different antibacterial are sodium piperacillin and doxycycline. Sodium piperacillin is a type of penicillin and doxycycline belongs to a class of drugs known as the tetracycline. Sodium piperacillin doxycycline Explain how penicillin are able to cure certain diseases caused by bacteria. Sodium piperacillin has a different side chain to the original penicillin developed by Florey and Chain. State one advantage of changing the side chain. E Explain why is may be necessary to give a mixture of several different types of antibacterial (such as penicillin and tetracycline) to patients suffering from diseases such as tuberculosis (TB) and MRSA (a disease caused by the presence of the staphylococcus aureus bacterium) D7 Antivirals 1. 2. 3. 4. 5. 6. 7. Describe the differences between bacteria and viruses, by referring to their structures and the way they multiply. Outline two ways in which antiviral drugs work. Suggest how acyclovir acts as an antiviral Describe two ways in which an antival drug can prevent the HIV virus from interacting with human cells Explain why effective treatment of AIDS with antiviral drugs is difficult Many diseases are the result of infection of the body by either bacteria or viruses. State the name of one disease caused by bacterial ______________ and viruses ____________ Discuss why viral infections are generally harder to treat than bacterial infections