Solutions Self-Assessment-Teacher Guide
advertisement

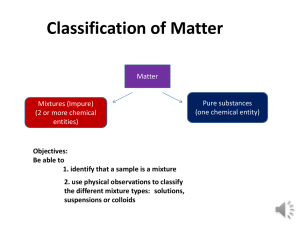
Physical Science Institute Summer 2013 Solutions Self-Assessment-Teacher Guide 1. B) Distinguish between types of aqueous mixtures (suspensions, colloids, and solutions) and realize that many properties of these mixtures are based on particle size. 2. D) Distinguish between types of aqueous mixtures (suspensions, colloids, and solutions) and realize that many properties of these mixtures are based on particle size. 3. B) Distinguish between types of aqueous mixtures (suspensions, colloids, and solutions) and realize that many properties of these mixtures are based on particle size. 4. B) Use diagrams to compare concentrations of solutions at the molecular level based on condition, i.e., unsaturated or saturated. 5. A) Know that volume is not conserved in solution formation and explain this using the kinetic model. 6. C) Know that volume is not conserved in solution formation and explain this using the kinetic model. 7. A) Use diagrams to compare concentrations of solutions at the molecular level based on condition, i.e., unsaturated or saturated. 8. C) Recognize that a saturated solution represents a dynamic equilibrium between the solute dissolving and precipitating. 9. C) Know that solubility of a solute in a solvent is a characteristic property and is related to attractive forces at the molecular level. 10. C) Know that solubility of a solute in a solvent is a characteristic property and is related to attractive forces at the molecular level. 11. B) Know that solutes, when dissolved, lower the freezing point of a solvent. 12. Know that volume is not conserved in solution formation and explain this using the kinetic molecular model. Since alcohol and water are mutually soluble and form a solution, the resulting volume of the mixture is less than the sum of the volumes of the individual liquids. Volume is NOT conserved in solution formation. In solution formation the alcohol molecules are interspersed between water molecules. The attractions between the water and alcohol molecules bring the molecules closer together in the solution than in the pure substances. This change in volume lowers the pressure in the sealed tube and allows the water and alcohol to vaporize and form bubbles. The composition of the bubble that results is a mixture of water and alcohol vapors. The hexane and water don’t form a solution and therefore there is no reduction in volume. 1


















![paper_ed6_31[^]](http://s3.studylib.net/store/data/007287934_1-3d5e82d0bd8f48a2cdb1e6fee9e0a30f-300x300.png)
