Audit and Review of Adults with Asthma on High Dose
advertisement

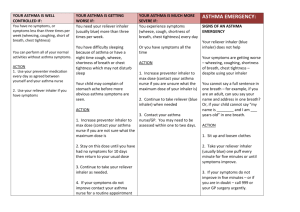
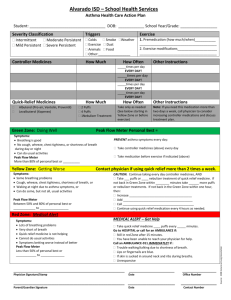
Audit and Review of Adults with Asthma on High Dose Inhaled Corticosteroids Background Inhaled corticosteroids (ICS) are safe and effective for the prevention of symptoms of asthma when used regularly at the recommended doses. The British Thoracic Society (BTS) and Scottish Intercollegiate Guidelines Network (SIGN) asthma guidelines recommend that regular inhaled corticosteroids are introduced if a patient requires short acting bronchodilator therapy more than twice a week, or if there are night-time symptoms more than once a week, or if there has been as exacerbation in the last 2 years. Regular use of inhaled corticosteroids reduces the risk of exacerbation of asthma. However, prolonged treatment with ICS at high doses carries a risk of systemic side effects such as adrenal suppression and a reduced bone mineral density predisposing patients to osteoporosis. There have been reports of a small risk of glaucoma and cataracts with prolonged high doses of inhaled corticosteroids, together with an increased risk of developing diabetes. Hoarseness, dysphonia, throat irritation, and candidiasis of the mouth or throat may occur. Paradoxical bronchospasm has been reported very rarely. Anxiety, depression, sleep disturbances, behavioural changes including hyperactivity, irritability, and aggression (particularly in children) have been reported; hyperglycaemia (usually only with high doses), cataracts, skin thinning and bruising have also been reported. High dose inhaled corticosteroids are introduced at step four of the BTS/ SIGN guidelines and are defined in the guidelines (given through metered dose inhaler) as: > 800micrograms beclometasone (Clenil Modulite®) > 400 micrograms beclometasone (Qvar®) > 800 micrograms budesonide > 400 micrograms fluticasone The BTS / SIGN asthma guidelines recommend stepping treatment down when asthma control is stable for at least 3 months to prevent over treating and to find and maintain at the lowest controlling step. The lowest possible dose of ICS which controls the symptoms of asthma should be used, where the dose should be reduced slowly, (consider a reduction every 3 months, decreasing the dose by 25 to 50% each time). Aim To optimise the use of high dose inhaled corticosteroids for asthma in adults and ensure that patients can use their device correctly and that they are being prescribed safely and effectively. Written by: Karen Homan, Sally-Jane Hamilton 2014, BCCG MMT Audit Objectives Patients with a repeat prescription for high dose inhaled corticosteroid have a documented indication for prescribing it. Patients have had an asthma review within the last year. Patients are able to use their inhalers correctly. Patients requiring high dose inhaled corticosteroid treatment are prescribed the lowest dose that controls their symptoms. Patients requiring high dose inhaled corticosteroid treatment are reviewed after three months of therapy. Patients using more than 12 short-acting relievers in the previous 12 months have an urgent review of their asthma control. Patients prescribed an ICS inhaler and a LABA inhaler separately should preferably be prescribed this as a combination inhaler. Patients requiring more than two courses of systemic steroids (oral or iv) in the previous 12 months or who require management using British Thoracic Society (BTS) stepwise treatment 4 or 5 to achieve control are identified and referred to a specialist asthma service. Patient Identification Search for all adult patients (over 12 years old) on a repeat prescription for high dose inhaled corticosteroid, including those on compound preparations such as Symbicort® and Seretide® (see Appendix 1 for SystmOne® search procedure). Data Collection See Appendix 2 for data collection spread sheet. Correct diagnosis recorded – Yes or No Asthma review within the last 12 months – Yes or No Inhaler technique check documented in the last 12 months Documented evidence of step down attempted in last 3 months- Yes or No Any evidence of symptoms of systemic side effects such as adrenal suppression, evidence of decreased bone mineral density (fractures) or glaucoma. Number of ICS inhalers (including ICS/LABA inhalers) issued in the past 12 months. Number of SABA inhalers issued in the past 12 months. ICS inhaler and LABA inhaler prescribed separately – Yes or No More than 2 courses of systemic steroids in previous 12 months Patients on step 4 or 5 of BTS who have not been referred to a specialist asthma service. Written by: Karen Homan, Sally-Jane Hamilton 2014, BCCG MMT Analysis Percentage of patients with correct diagnosis recorded. Percentage of patients with an asthma review within the last 12 months. Percentage of patients that have had their inhaler technique checked and documented. Percentage of patients on high dose inhaled corticosteroid with documented evidence of step down attempted in last three months. Percentage of patients with symptoms of ICS adverse effects such as low bone mineral density, diabetes, glaucoma or adrenal suppression. Percentage of patients with less than 12 ICS or ICS/LABA inhalers in the last 12 months. Percentage of patients using less than 4 SABA inhalers in past 12 months. Percentage of patients using more than 12 SABA inhalers in past 12 months. Percentage of patients prescribed an ICS inhaler and a LABA inhaler as separate inhalers. Percentage of patients prescribed more than 2 courses of systemic steroids (oral or iv) in the previous 12 months. Percentage of patients on step 4 or 5 of BTS who have not been referred to a specialist asthma service. Possible Action Plan For each patient, record what action is required. Some suggested actions include: If incorrect diagnosis recorded, then read code diagnosis. If no review within the last 12 months, review patient. If inhaler technique has not been checked, review patient. If patient on high dose inhaled corticosteroid with no documented evidence of step down attempted in last three months, review patient and step down if clinically appropriate. If patient using less than 12 ICS or ICS/LABA inhalers in the last 12 months, review patient and consider suitability for stepping down ICS or whether they are adhering to their preventer therapy. If patient not using SABA, consider suitability for stepping down inhaled corticosteroid if patient is on one. If patient has used more than 12 SABA inhalers in the past 12 months, urgently review patient as asthma may not be controlled. If patient is on a separate ICS inhaler and LABA inhaler, review patient and prescribed ICS / LABA combination inhaler if both components are required. If patient is experiencing side effects, review treatment. If patient has required more than 2 courses of steroids in the previous 12 months or at step 4 or 5 of BTS and NOT been referred to a specialist asthma service, make referral. Written by: Karen Homan, Sally-Jane Hamilton 2014, BCCG MMT References BNF May 2014 accessed on-line at www.medicinescomplete.com user registration required. British Guideline on the Management of Asthma. The British Thoracic Society and Scottish Intercollegiate Guidelines network. May 2008, revised May 2011. Accessed via www.brit-thoracic.org.uk Why asthma still kills. The National Review of Asthma Deaths (NRAD). Royal College of Physicians. May 2014. Written by: Karen Homan, Sally-Jane Hamilton 2014, BCCG MMT Appendix 1 Running the SystmOne® Search 1. In SystmOne enter Clinical Reporting Clinical Reporting) (in the top toolbar select Reporting Searching for the high dose inhalers: 2. Click ‘New’ to open a new search. Give the search a name. 3. From the left hand menu select Demographics Age 4. Tick the box ‘Current age’ and enter Over 12 Years 5. From the left hand menu select Clinical Repeat Templates Written by: Karen Homan, Sally-Jane Hamilton 2014, BCCG MMT 6. Then select Exact drugs, and click on the brown bottle icon 7. The ‘Select Multiple Drugs or Appliances’ window opens. In the search box type the first few letters of the drug name you are searching for and press Enter 8. Click on the drug you want to select to highlight it and click on the black arrow to move it into the right hand pane 9. Continue to select all the drugs and move them into the right hand pane. Make sure you search both brand and generic: Beclometasone 250mcg inhalers Asmabec Clickhaler 250mcg Clenil 250mcg Beclometasone 400mcg inhalers Pulvinal 400mcg Becodisks 400mcg Budesonide 400mcg inhalers Pulmicort 400mcg Easyhaler budesonide 400mcg Budesonide/Formoterol Inhaler 400/12 Symbicort® Turbohaler 400mcg/12mcg Fluticasone 125mcg inhalers Fluticasone 250mcg inhalers Fluticasone 500mcg inhalers Flixotide 125mcg Evohaler Flixotide 250mcg Evohaler Flixotide 250mcg Accuhaler Flixotide 500mcg Accuhaler Fluticasone/Formoterol Inhaler 250/10mcg Flutiform® Inhaler 250/10mcg Fluticasone/Salmetrol Inhaler 125/25mcg Fluticasone/Salmeterol Inhaler 250/25mcg Fluticasone/Salmeterol Inhaler 500/50mcg Fluticasone/Salmeterol Inhaler 250/50mcg Seretide® 125 Evohaler 125mcg/25mcg Seretide® 250 Evohaler 250mcg/25mcg Seretide® 500 Accuhaler 500mcg/50mcg Written by: Karen Homan, Sally-Jane Hamilton 2014, MMT Seretide® 250BCCG Accuhaler 250mcg/50mcg Fluticasone /Vilanterol Inhaler 92µg/22µg Relvar® Ellipta® 92µg/22µg Fluticasone /Vilanterol Inhaler 184µg/22µg Relvar® Ellipta® 184µg/22µg 10. When you have selected all the drugs click OK to close the Selection window 11. Click OK to complete & close the search To join the search to the Asthma Register: 12. Click ‘New’ to open a new search. Give the search a name 13. In the left hand pane select Report Joining Join to two reports 14. Click on ‘Select report one’ 15. Locate your search run above, and double click on it to select it 16. Click on ‘Select report two’ 17. Locate the Asthma register (System Wide QOF Asthma AST001 Register) and double click on it to select it Written by: Karen Homan, Sally-Jane Hamilton 2014, BCCG MMT 18. Back in the search window select the first join type: (This will search for patients on the selected drugs AND on the Asthma register) 19. Click OK to complete & close the search 20. Finally, click on the green triangle to run the search to view the patients Written by: Karen Homan, Sally-Jane Hamilton 2014, BCCG MMT , and the magnifying glass Appendix 2 Data Collection Template - High Dose Inhaled Corticosteroids in Adult Asthmatics Audit Practice ………………… Number of patients reviewed…………Audit carried out between …/…/2014 and …/…/2014 Audit lead ……………………… Patient ID Medication details Indication for ICS inhaler in patient notes? Asthma review in the last 12 months Inhaler technique check documented? Yes or No Step down attempted in the past 3 months? ICS inhaler Yes Yes Yes Yes Dose No No No No Any side effects reported (e.g. symptoms of adrenal suppression, low bone mineral density, glaucoma, diabetes) Number of ICS inhalers (include ICS/LABA combinations) issued in past 12 months? Number of SABA inhalers issued in past 12 months? Are LABA and ICS inhaler prescribed separately? Yes or No More than 2 courses of systemic steroids (oral or iv) in previous 12 months? Yes or No If patient Action on step 4 Required or 5 of BTS, have they been referred to a specialist asthma service? Yes or No Yes Yes Yes No No No Appendix 3 Example Patient Information Letter Practice Address Tel: Fax: Date ~[Title/Initial/Surname] ~[Patient Address Block] Dear ~[Title]~[Surname], We would like to invite you to come for an asthma review as we are currently looking at the medication we use to treat asthma where a high dose inhaled corticosteroid preventer inhaler has been prescribed. National recommendations advise us to review asthma patients regularly to check such things as how well the asthma is controlled, inhaler technique, how often inhalers are used, any side effects experienced, whether treatment can be stepped down. Please book an appointment to see the asthma nurse at your earliest convenience. Yours sincerely Practice Nurse