SKEMA SOLAF2 KERTAS3
advertisement
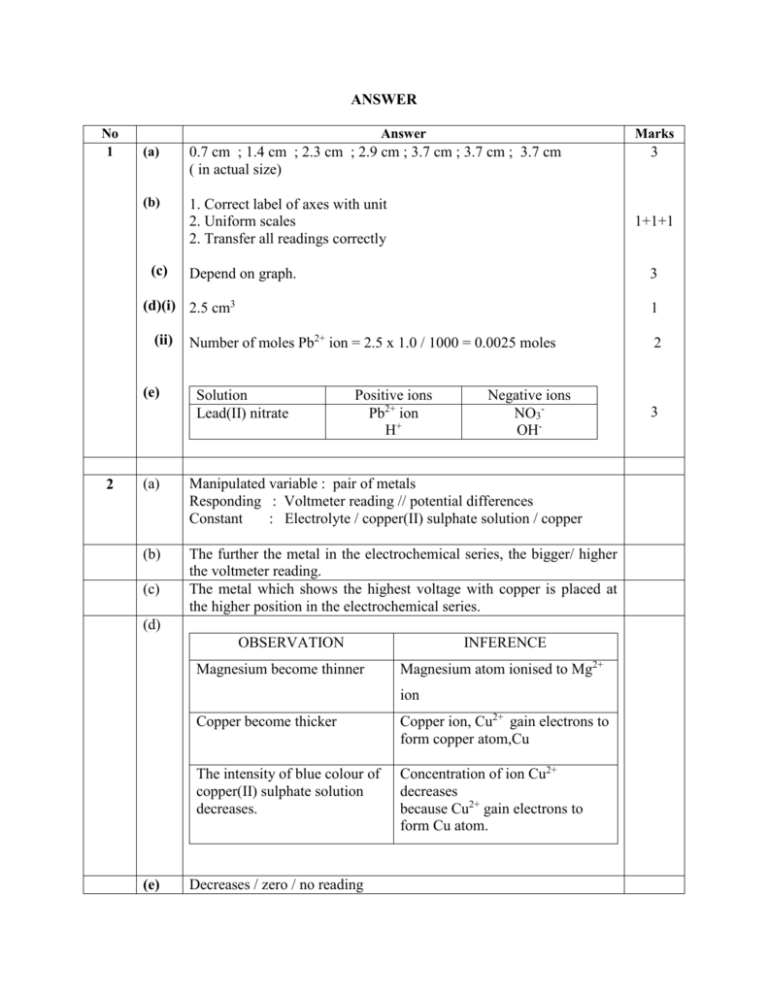
ANSWER No 1 Answer Marks (a) 0.7 cm ; 1.4 cm ; 2.3 cm ; 2.9 cm ; 3.7 cm ; 3.7 cm ; 3.7 cm ( in actual size) (b) 1. Correct label of axes with unit 2. Uniform scales 2. Transfer all readings correctly (c) 1+1+1 Depend on graph. 3 (d)(i) 2.5 cm3 (ii) (e) 2 1 Number of moles Pb2+ ion = 2.5 x 1.0 / 1000 = 0.0025 moles Solution Lead(II) nitrate Positive ions Pb2+ ion H+ Negative ions NO3OH- (a) Manipulated variable : pair of metals Responding : Voltmeter reading // potential differences Constant : Electrolyte / copper(II) sulphate solution / copper (b) The further the metal in the electrochemical series, the bigger/ higher the voltmeter reading. The metal which shows the highest voltage with copper is placed at the higher position in the electrochemical series. (c) (d) OBSERVATION Magnesium become thinner INFERENCE Magnesium atom ionised to Mg2+ ion (e) 3 Copper become thicker Copper ion, Cu2+ gain electrons to form copper atom,Cu The intensity of blue colour of copper(II) sulphate solution decreases. Concentration of ion Cu2+ decreases because Cu2+ gain electrons to form Cu atom. Decreases / zero / no reading 2 3 Question 3 (a) 3 (b) 3 (c) Rubric [ Able to state the aim accurately ] Example : To identify liquid X and Y / hexane and hexene using chemical test. Score 3 [Able to state the aim less accurately ] Example : To identify liquid X and Y / hexane and hexene. 2 [Able to state the idea of problem statement] Example : To compare liquid X and Y / hexane and hexene 1 [No response given or wrong response] 0 [ Able to state the three variables correctly] Example : Manipulated variable : Liquid X and liquid Y // Hexane and hexene Responding variable : The change in colour Controlled variable : Volume of liquid X and Y // Hexane and hexene, Volume of bromine in 1,1,1trichloroethane// Volume of acidified potassium manganate (VII) solution. 3 [Able to state any two variables correctly ]] 2 [Able to state any variable correctly] 1 [No response given or wrong response] 0 [ Able to state the relationship between the manipulated variable and the responding variable] Example : Hexene will decolourise brown colour of bromine in 1,1,1trichloroethane// Purple colour of acidified potassium manganate (VII) solution. Hexane will not decolourise brown colour of bromine in 1,1,1trichloroethane // purple colour of acidified potassium manganate (VII) solution. 3 Question Rubric [Able to state the hypothesis of the experiment less accurately] Example 1 : Hexene will decolourise bromine and hexane will not decolourise bromine (without colour) Score 2 Example 2 : Hexene will decolourise brown colour of bromine // purple colour of acidified potassium manganate (VII) solution. OR Hexane will not decolourise brown colour of bromine // purple colour of acidified potassium manganate (VII) solution. 1 [Able to state an idea of hypothesis] Example : Hexene is reactive than hexane // Hexene will react with bromine but hexane is not. 0 [ No response given or wrong response] 3(d) [Able to list the materials and apparatus accurately] Example : Material : acidified potassium manganate(VII) solution // Bromine in 1,1,1-trichloroethane, liquid Y and Z // Hexane and hexene Apparatus : test tube,dropper ,stopper 3 [Able to list incomplete materials and apparatus for the experiment] Example : Material : Acidified potassium manganate (VII) solution //Bromine, 2 liquid Y and Z Apparatus : test tube, dropper [Able to list the minimum material and apparatus correctly] Example : Material : Bromine, liquid Y and liquid Z 1 Apparatus: test tube Question 3(e) Rubric [Able to state the following steps correctly] Sample answer : 1. About 2 cm3 of hexane/ liquid Y is poured into a test tube. 2. Three drops of bromine in 1,1,1-trichloroethane // 2 cm3 acidified potassium manganate (VII) solution are added into the test tube 3. The test tube is closed with a stopper 4. The mixture is shaken. 5. The observation is recorded. 6. Steps 1-6 are repeated using liquid Z / hexene [Able to write the necessary steps of the experiment] Steps 1,2,4 and 6 3(f) Score 3 2 [Able to write the minimum steps of the experiment] Steps 1,2 and 6 1 [No response given or wrong response] 0 [Able to tabulate the data which includes the following two information] 1. Heading for the manipulated variable 2. Heading for responding variable Example : Liquid 2 Observation Y / Hexane Z / Hexene [Able to exhibit the tabulation of data but not completely labeled] 1 Example : Liquid Y / Hexane Z / Hexene No response given or wrong response 0