lab report
advertisement

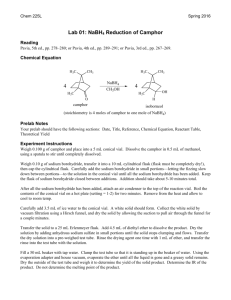
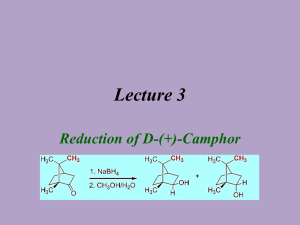
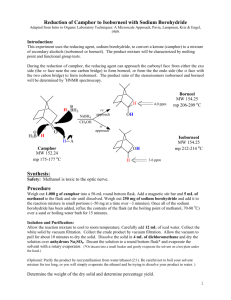
Abby Bacon, Chem 213 Synthetic #2 FFR TA: Brandon Smith June 29, 2012 Experiment 32: Reduction of Camphor Introduction Camphor is a versatile compound found in plants. It is classified as a “bicyclic monoterpene ketone” and is used in things ranging from moth repellent to anti-infective/anti-itch topical ointments, embalming fluids, and even Vicks Vaporub. It is found naturally in the wood of the Cinnamomum camphora tree, but can also be made synthetically from the fractional distillation of turpentine oil. Camphor is well known for its menthol and cedar-like scent, and can be fatal if consumed in high doses1. While camphor itself has many everyday uses, it can also be reduced to ()-Borneol and ()-Isoborneol, both of which serve many purposes as well. ()-Borneol can be found in essential oils and is thought to have many pharmacological applications2. ()-Isoborneol is also found in essential oils and is thought to have antioxidant properties3. In chemistry, reduction reactions are very common, and include any reaction that results in an increased electron density on a carbon atom. This includes breaking a bond between carbon and a more electronegative atom, like oxygen or nitrogen, or forming a bond between carbon and a less electronegative atom, like hydrogen. Reduction reactions require the help of a reducing agent, most commonly sodium borohydride (NaBH4) or lithium aluminum hydride (LiAlH4). When given a choice, NaBH4 is generally selected due to the fact that it is soluble in water, and less reactive than LiAlH4 making it safer to use. NaBH4 is most often used to convert ketones and aldehydes to alcohols, like in this reaction, while LiAlH4 is most often used to reduce esters, carboxylic acids, and amides4. Bacon 2 Reduction of carbonyl compounds, which include ketones like camphor, is the most common reaction used to prepare alcohols, like ()-borneol and ()-isoborneol4. In the case of camphor reduction, a C-H bond is formed, as well as an O-H bond, yielding a secondary alcohol. The mechanism for this reaction is as follows: (a) Production of ()-Borneol (b) Production of ()-Isoborneol In this reaction, NaBH4 acts as a hydride donor in a nucleophilic addition reaction and attacks the carbon atom, breaking the C=O bond and leaving a negative charge on the oxygen. The oxygen is then protonated by H3O+ yielding a secondary alcohol. In (a) the hydride attacks from the top to form ()-borneol, while in (b), the hydride attacks from the bottom to form ()-isoborneol. Due to the steric hindrance of the –CH3 groups on the top of the molecule, ()-isoborneol is more likely to be produced as the hydride ion can more easily attack from the bottom. For that reason, it is predicted that ()-isoborneol will be the majority product in this experiment. Bacon 3 The overall purpose of this experiment is to synthesize ()-borneol and ()-isoborneol via the reduction of camphor, and then isolate and purify the product using vacuum filtration. NMR, IR, GC, GC-MS, and melting point data were all gathered to analyze the product. Experimental ()-Borneol and ()-Isoborneol. Camphor (0.201 g, 1.32 mmol) and methanol (2 mL) were added to a 10 mL round bottom flask and stirred at room temperature with a ½” magnetic stir bar until dissolved. Sodium borohydride (0.123 g, 3.25 mmol) was added gradually while stirring yielding a clear liquid. After all sodium borohydride was added, an air condenser was affixed to the flask and the mixture was refluxed at 68ºC in a sand bath for thirty minutes. The mixture was then allowed to cool to room temperature and ice water (3.5 mL) was added. The solid was isolated by vacuum filtration and washed with cold water (2 x 5 mL) to yield white crystals (0.116 g, 57.0%); 1H NMR (60 MHz, CDCL3) 3.649 (s, 1H), 1.395-1.697 (m, 4H), 0.6561.033 (m, 9H); IR max 3337.5, 2945.9, 2876.1; MP ran to 200ºC, sublimed; GC (phenyl menthylpolysiloxane, 40ºC to 250ºC at 10ºC/min) RT 7.410; GC-MS (phenyl menthylpolysiloxane, 40ºC to 250ºC at 10ºC/min) 13.32 min, m/z 94.98, 13.48 min, m/z 94.98. Results and Discussion The synthesis of ()-borneol and ()-isoborneol was carried out by the reduction of camphor. Using NaBH4, the carbon of the ketone on camphor was attacked by a hydride, and then the oxygen was protonated to yield a secondary alcohol. Looking at percent yield shows the experiment was successful in synthesizing the desired product. A total of 116 mg was synthesized, which is a 57.0% yield. This yield is just above half of the theoretical yield, which allowed for enough product to be collected in order to run further tests like melting point, NMR, IR, GC, and GC-MS. Several problems could have Bacon 4 resulted in the loss of product including product boiling away during refluxing or some product remaining in solution after cooling to room temperature and then placing in ice water. Some product may also have been lost due to human error or in transferring the product. The 1H NMR spectrum (Figure 1, Supplemental Information) helps to illustrate the exact composition of the product. Overall, four distinct signals are evident: three from ()-borneol and ()-isoborneol and one from an impurity. The multiplet of peaks at 0.656-1.033 ppm with an integral value of 9.00 corresponds to the nine protons of the methyl groups of ()-borneol and ()-isoborneol. The multiplet of peaks at 1.395-1.697 ppm with an integral value of 4.11 corresponds to the six secondary hydrogens of ()-borneol and ()-isoborneol. The singlet at 3.649 with an integral value of 0.37 corresponds to the hydrogen of the alcohol group and the secondary hydrogen on that same carbon. Because the NMR is a mixture of two compounds, it is difficult to determine the exact number of protons shown; therefore, the integral values may be slightly lower or higher than expected. Instead, it is more relevant to look at how upfield or downfield the peaks are to determine the type of hydrogens illustrated. The remaining peak shows that there was a contaminant present in the sample. This peak is at 7.279 ppm with an integral value of 0.03 that most likely corresponds to d-chloroform which appears at 7.26 ppm. Because the integral value is so low, it can be assumed that very little of the contaminant was present in the sample. The IR spectrum (Figure 2, Supplemental Information) allows additional information as to the exact composition of the product. The signal at 3337.4 cm-1 corresponds to the –OH stretch of ()-borneol and ()-isoborneol, and the signals at 2945.9 and 2876.1 cm-1 correspond to the C-H stretch. Most notably, there is no signal at 1715 cm-1 that would correspond to the Bacon 5 ketone of camphor. This shows that the reaction was successful in producing ()-borneol and ()-isoborneol and that no starting material was left in the sample. A melting point was taken for the product but the results were relatively inconclusive. ()-Borneol and ()-isoborneol typically melt at 206-209ºC and 212-214ºC respectively. Despite sealing the melting point tube with parafilm, however, the sample still sublimed around 200ºC. Due to the high melting point, it is very difficult to obtain accurate results using the Mel-Temp. The sample did not melt at or before 180ºC though (the melting point of camphor), once again pointing to the fact that very little or no starting material remained to cause melting point depression. Finally, GC (Figure 3, Supplemental Information) and GC-MS (Figure 4, Supplemental Information) were taken to determine the exact composition of the final product. Because the reduction of camphor yields both ()-borneol and ()-isoborneol, it is important to analyze the percent composition to report the majority product. Looking at the GC, three peaks are evident. The first two peaks can be disregarded, however, as they correspond to the solvent and dichloromethane. The remaining peak is shown in Table 1. Table 1: GC Data for ()-Borneol and ()-Isoborneol Component RT Area 7.410 4154 ()-Isoborneol % Composition 100.0 This peak has a retention time of 7.410 min and has an area of 4154. Because it is the only peak of importance, it accounts for 100% composition of the product. Looking carefully shows that there is a smaller peak after this peak, but it is too small to be annotated on the GC. A GC-MS was then taken to further analyze the exact composition. The results of this test are show in Table 2. Bacon 6 Table 2: GC-MS Data for ()-Borneol and ()-Isoborneol Component RT m/z 13.32 94.98 ()-Isoborneol 13.48 94.98 ()-Borneol This time there are two peaks of importance. The first peak has a retention time of 13.32 minutes and an m/z value of 94.98. This peak is then identified by the library as ()-isoborneol. The second peak has a retention time of 13.48 minutes and an m/z value of 94.98. This peak is then identified by the library as ()-borneol. Comparing the pattern of these peaks to those observed on the GC, ()-isoborneol is identified as the larger peak (RT 7.410 min), while ()-borneol is the smaller peak that could not be annotated. This supports the original hypothesis that predicted ()-isoborneol would be the majority product formed due to lack of steric hindrance. Conclusion Reduction reactions are very common in the field of chemistry. The reduction of camphor to yield ()-borneol and ()-isoborneol is a good example of this type of reaction, and this lab helped to illustrate the basic understanding behind determining the major product synthesized in a given reaction. Overall, the percent yield showed that the desired products were successfully synthesized, and the data from NMR and IR support this as well. Minimal contamination was seen in the spectra, which also showed that the reaction proceeded as expected. The results obtained from GC and GC-MS illustrate the exact composition of the products, confirming that ()-isoborneol was the majority product while ()-borneol was the minority product. Overall, the reduction of camphor was successful. Bacon 7 References (1) National Center for Biotechnology Information; U.S. National Library of Medicine, Camphor. PubChem. [online] 2009. http://pubchem.ncbi.nlm.nih.gov/summary/summary. cgi?cid=2537#x94 (accessed June 27, 2012). (2) Horvathova, Eva, et al.; Oxford University Press, Borneol Administration Protects Primary Rat Hepatocytes Against Exogenous Oxidative DNA Damage. Oxford Journals. [online] 2012. http://mutage.oxfordjournals.org/content/early/2012/04/26/mutage. ges023.abstract (accessed June 27, 2012). (3) Tian, LL, et al. National Center for Biotechnolody Information; U.S. National Library of Medicine, Protective Effect of ()-Isoborneol Against 6-OHDA-induced Apoptosis in SH-SY5Y cells. PubMed. [online] http://www.ncbi.nlm.nih.gov/pubmed/17975304 (accessed June 27, 2012). (4) McMurry, John. Organic Chemistry, 7th ed.; Brooks/Cole: California, 2008; pp 348-349, 609-611, 708-709.