TRANS-MARA WEST ASSESSMENT TEST 233/2 CHEMISTRY
advertisement
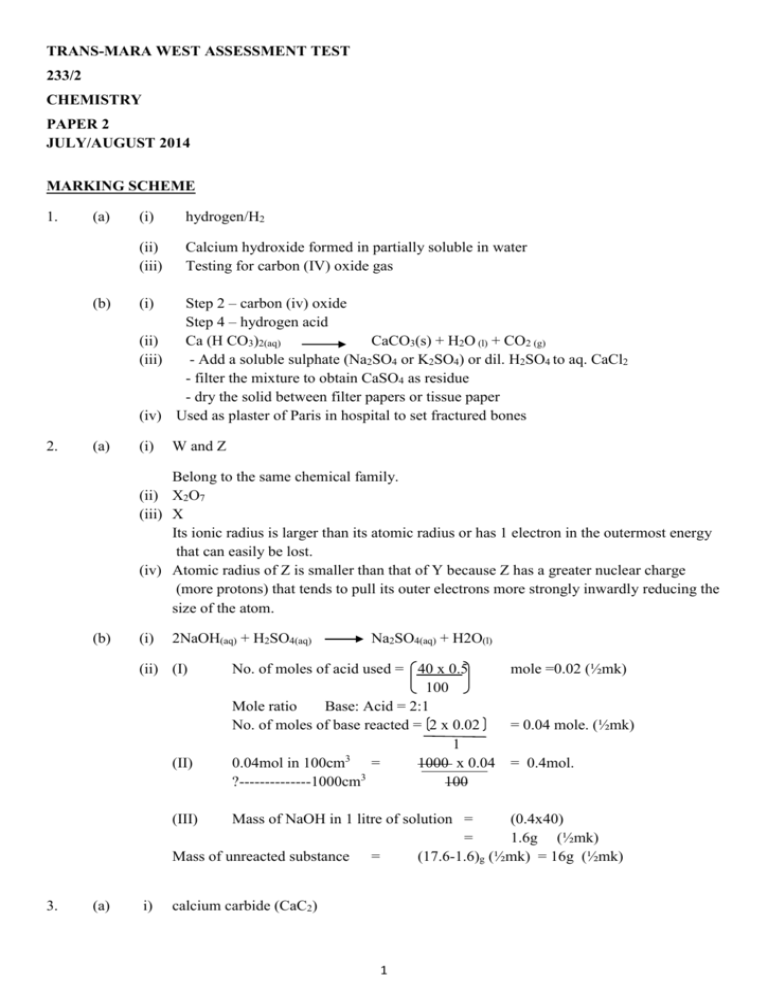
TRANS-MARA WEST ASSESSMENT TEST 233/2 CHEMISTRY PAPER 2 JULY/AUGUST 2014 MARKING SCHEME 1. 2. (a) (i) hydrogen/H2 (ii) (iii) Calcium hydroxide formed in partially soluble in water Testing for carbon (IV) oxide gas Step 2 – carbon (iv) oxide Step 4 – hydrogen acid (ii) Ca (H CO3)2(aq) CaCO3(s) + H2O (l) + CO2 (g) (iii) - Add a soluble sulphate (Na2SO4 or K2SO4) or dil. H2SO4 to aq. CaCl2 - filter the mixture to obtain CaSO4 as residue - dry the solid between filter papers or tissue paper (iv) Used as plaster of Paris in hospital to set fractured bones (b) (i) (a) (i) W and Z Belong to the same chemical family. (ii) X2O7 (iii) X Its ionic radius is larger than its atomic radius or has 1 electron in the outermost energy that can easily be lost. (iv) Atomic radius of Z is smaller than that of Y because Z has a greater nuclear charge (more protons) that tends to pull its outer electrons more strongly inwardly reducing the size of the atom. (b) (i) 2NaOH(aq) + H2SO4(aq) (ii) (I) (II) Na2SO4(aq) + H2O(l) No. of moles of acid used = 40 x 0.5 mole =0.02 (½mk) 100 Mole ratio Base: Acid = 2:1 No. of moles of base reacted = 2 x 0.02 = 0.04 mole. (½mk) 1 3 0.04mol in 100cm = 1000 x 0.04 = 0.4mol. 3 ?--------------1000cm 100 (III) Mass of NaOH in 1 litre of solution = (0.4x40) = 1.6g (½mk) Mass of unreacted substance = (17.6-1.6)g (½mk) = 16g (½mk) 3. (a) i) calcium carbide (CaC2) 1 (ii) Conc. Sulphuric (IV) acid/conc. H2SO4 (iii) - C ≡ C (iv) Step 3 = polymerization Step 5 = hydration Step 6 = hydrogenation (v) Preparation of margarine (hardening of oil into fats) (vi) Step 2 = C2H2 (g) +HCl(g) C2H3Cl(g) (ignore state symbols, accept structural formulae) (b) 4 C2H2 (g) + H2 (g) C2H4 (g) (Condition as in step 2apply) (i) (I) K1 and K3 (II) K2 and K5 (ii) K4 (iii) 2CH3 CH2 CH2 C – OH +2k 2CH3 CH2 CH2 COOK + H2 (a) this is the energy change that occurs when a substance is formed from its substituent elements. (b) (i) - enthalpy of combustion of hydrogen gas (1mk) - enthalpy of formation of water. (1mk) (ii) 2CO2 (g) +3H2O (l) H=+ 1560Kj/mol Energy C2H(aq)7/2O2(g) Reaction progress 2C + 3H2 (g) C2H6(g) (iii) Hf 7 +4O2 3O2 H2 H3 2CO2 (g) + 3H2O (l) 2 /2O2 H1 Hf + H1 = H2 + H3 Hf =H2 + H3 - H1 Hf = (-394) + 2 (-286) – (-1560) kJ Hf = (-788 -572) kJ-((-1360 +1560kJ) +200kJ (c) (i) H=MC = 500 kg x 4.2Kj x 21.5K 1000 Kg = (0.5 x 4.2 x 21.5Kj) = (ii) 6. (a) i) ii) (b) i) ii) (a) (c) i) (a) ii) (b) 45.15Kj 30g ethane evolves 1560Kj ?g ------------------ 45.15kJ = 45.15k x 30 1560 5. T 45.15kJ x 30g 1560kJ = 0.868g A and C Ba2+(aq) + SO2-4(aq) BaSO4(s) Pb2+(aq) + SO2-4(aq) PbSO4 Magnesium stearate /scum/magnesium octadecanate volume of soap solution required to form lather is the lowest before and after boiling. (b) C-volume of soap solution required to form lather reduced after boiling. Addition of Na2CO3 Distillation Use of ion exchange resins Addition of Ca (OH)2 Bauxite Iron Silicon i) 3 ii) I) - High temperature is expensive to attain and maintain OR - Al2O3 is a very poor conductor of electricity at very high temperature. (II) Adding cryolite (impurity) to molten aluminium oxide - Denser than the electrolyte - Insoluble in the electrolyte iii) Al3+(aq) + 3e Al(aq) Q=It Q= (40,000 X 60 X 60) C=144000000C (c) 3F -----------------------27g Al (3 x 96500) c ---------- 27g Al. 144000000c? = 7 144000000 x 27 3x96500 g = 144000000 x 27 289500 ≈ 13.43kgAl = 13430g (a) - Ammoniacal brine/ammoniate brine Sodium hydrogen carbonate/NaHCO3 Ammonium chloride/NH4Cl Calcium chloride/CaCl2 (b) 2NH4Cl (aq) + Ca (OH) 2(aq) NaHCO3(s) (c) (d) (e) - heat CaCl2 (aq) +2H2O (l) + 2NH3 (g) Na2CO3(s) + H2O(l) + CO2(g) Filtration Decomposition Limestone/Calcium carbonate - Pump sea water to shallow basins/bonds Evaporation of water takes place Sodium chloride crystallizes out. 4