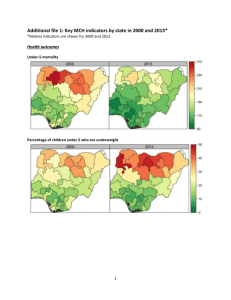
Immunization: DFM Policy & Procedure Manual
advertisement

DFM Policy & Procedure Manual □ Clinical Operations □ Faculty Council □ Administrative X□Other Effective Date: 1/1/2011 X□ Original Page __1____ __ 7_ Policy # □Revision Title: Immunization Policy PURPOSE The purpose of this policy is to uphold: Recommended Immunization Schedules for Persons Aged 0 through 18 Years as approved by the American Academy of Pediatrics (AAP), the Advisory Committee on Immunization Practices (ACIP)and the American Academy of Family Physicians (AAFP). Recommended Adult Immunization Schedule as approved by the Advisory Committee on Immunization Practices (ACIP), the American Academy of Family Physicians (AAFP), the American College of Obstetricians and Gynecologists (ACOG), and the American College of Physicians (ACP) DFM staff will promote and follow the current Recommended Immunization Schedules, unless otherwise instructed by the UW Health Immunization Task Force. PRINCIPLES The UW Health Immunization Task Force discourages any practice that deviates from the ACIP Recommended Immunization Schedules. Vaccination providers should adhere to the following immunization principles: INTERVALS: Intervals between doses of multidose antigens provide optimal protection and/or have the best evidence of efficacy. AGE: Vaccines are recommended for members of the youngest age group at risk for experiencing the disease for whom efficacy and safety have been demonstrated. Delaying administration of vaccines leaves patients at risk for infection during their most vulnerable ages. SIMULTANEOUS ADMINISTRATION: Simultaneously administering all vaccines for which a person is eligible is critical, because simultaneous administration increases the probability that a child will be vaccinated fully at the appropriate age. 1 DFM Policy & Procedure Manual □ Clinical Operations □ Faculty Council □ Administrative X□Other Effective Date: 1/1/2011 X□ Original □Revision Page __2____ __ 7___ Policy # Title: Immunization Policy COMBINATION VACCINES: Use of licensed combination vaccines is preferred to separate injection of their equivalent component vaccines to reduce the number of injections and missed opportunities. ALTERNATE PRODUCTS: Medication errors and patient safety are of increasingly concern and every effort must be made to prevent them. Stocking multiple brands of the same or similar medications increases the likelihood of errors. INTERCHANGEABILITY: Certain vaccines are available from different manufacturers, and these vaccines usually are not identical. Whenever possible, the same brand of vaccine should be used for all doses of the vaccination series. If vaccination providers do not know or have available the type of vaccine previously administered to a child, any age-appropriate vaccine containing the appropriate antigens should be used to continue or complete the series. LAPSES: Longer-than-recommended intervals between doses do not reduce final antibody concentrations. An interruption in the vaccination schedule generally does not require restarting the entire series of a vaccine. VACCINATION STATUS: Providers should only accept written, dated records as evidence of vaccination. Self-reported doses of vaccine without written documentation should not be accepted. If records cannot be located, these persons should be considered susceptible and should be started on the age-appropriate vaccination schedule. Serologic testing for immunity is an alternative to vaccination if there is a history of disease. VIS: CDC Vaccine Information Statements must be provided for all vaccines offered and an opportunity to ask questions offered. REFUSALS/DECLINATION DOCUMENTATION: A limited number of persons will have religious or personal objections to vaccinations. For patients and parents who refuse immunization for other reasons (personal conviction waivers), declination/responsibility refusal forms should be used. For providers who do choose to offer alternative immunization schedules, obtaining informed consent from families indicating that they have been counseled on the recommended schedule and declining to follow those recommendations is advised. Parents should be advised of state laws pertaining to school or child-care entry, which might require that unvaccinated children be excluded from school or child care during outbreaks. DFM Policy & Procedure Manual 2 □ Clinical Operations □ Faculty Council □ Administrative X□Other Effective Date: 1/1/2011 X□ Original □Revision Page __3____ __ 7__ Policy # Title: Immunization Policy STANDARDS DFM staff will adhere to the CDC/ACIP/AAP Standard for Childhood Immunizations (Appendix 1) and as distinguished below in the components of accessibility, education, vaccine precautions, administration, documentation and follow up. ACCESSIBILITY: All children will have every opportunity and encouragement to receive immunizations in accordance with the Recommended Immunization Schedules for Persons Aged 0 through 18 Years. Accessibility to these vaccines may include the Vaccines for Children Program (VFC), a federally funded vaccine supply program in which the providers throughout UWMF participate. In this program vaccines are provided through the Wisconsin Immunization Program to public and private providers to vaccinate eligible children at no cost to providers. Eligible children for the VFC program are those from birth through 18 years old who: a) Are eligible for Medicaid, b) Have no health insurance c) Are Native American or Alaska Native, d) Have health insurance that does not cover immunizations. A supplemental component of the VFC program includes the availability of free hepatitis B vaccine to all newborn infants, regardless of payor status, who are delivered at VFC participating birthing hospitals where DFM physicians practice. EDUCATION: Education about the use of immunizations and their benefits and risks will be provided to the parent or legal guardian of any child to whom the provider intends to administer a vaccine. This includes the use of, but is not limited to, the federally mandated use of Vaccine Information Statements (VIS). Vaccine Information Statements (VIS’s) are information sheets produced by the CDC to explain vaccines to recipients, their parents, or their legal guardian. VIS’s explain the benefits and risks of a vaccine and are required by Federal law to be given out either before each dose of a vaccine covered under the National Childhood Vaccine Injury Act or by a mechanism approved by the Attorney General of the State of Wisconsin. CLINICAL STAFF EDUCATION: Clinical staff must maintain currency of an immunization services knowledge base that includes the review of the following immunization curriculum: Teaching Immunization Delivery and Evaluation http://www2.edserv.musc.edu/tide/menu.lasso and/or Understanding the Basics: General Recommendations on Immunization 2005 http://www.cdc.gov/vaccines/ed/youcalltheshots.htm 3 DFM Policy & Procedure Manual □ Clinical Operations □ Faculty Council □ Administrative X□Other Effective Date: 1/1/2011 X□ Original Page __4____ __ 7_ Policy # □Revision Title: Immunization Policy VACCINE CONTRAINDICATIONS AND PRECAUTIONS See Guide to Contraindications and Precautions to Commonly Used Vaccines (Immunization Action Coalition) DFM staff will screen prospective vaccine recipients for the following conditions and consult with the provider if these exist: Severe allergic reaction (e.g. anaphylaxis) after a previous vaccine dose or to a vaccine component. Pregnancy Known severe immunodeficiency (hematologic and solid tumors; receiving chemotherapy; congenital immunodeficiency; long-term immunosuppressive therapy,; or patients with HIV infection who are severely immunocompromised. Moderate to severe acute illness with or without fever. History of GuillainBarre Syndrome (GBS) within 6 weeks after a previous dose of vaccine. PROCEDURE: VACCINE ADMINISTRATION 1. Physician, PA, NP must be present in the clinic before administering immunizations. 2. Obtain a patient’s past immunization history by referring to the patient’s clinical record and Wisconsin Immunization Registry (WIR). 3. Obtain/review a history of allergies or other disease conditions proper to initiation of immunization of any patient; if there is a history of allergies in the family (i.e. latex) or of previous reaction to the same immunization, refer the patient to a physician or provider. 4. Provide a patient or responsible party the appropriate Vaccine Information Statement (VIS) from the Centers for Disease Control, or provide the equivalent information in another form if necessary. 5. Inform parents, guardians, legal representatives, and adolescent and adult patients about the benefits and risks of vaccines in an understandable language. Provide an opportunity for questions and the provision of answers by the nurse or physician before each vaccination. 4 DFM Policy & Procedure Manual □ Clinical Operations □ Faculty Council □ Administrative X□Other Effective Date: 1/1/2011 X□ Original Page __5____ __ 7__ Policy # □Revision Title: Immunization Policy 6. Administer immunizations according to a provider’s active order or by protocol.. 7. Administer immunizations using standard precautions and vaccine administration procedures, including the use of OSHA approved needle protection systems. 8. Follow specific instructions of each vaccine. Check expiration date, appearance, and color of vaccine, if appropriate. Review package inserts regarding dosage, administration (including specifications for needle length for SQ or IM injections) and contraindications for immunization. 9. Prepare skin area appropriately if immunization will be injected. If immunization is delivered via oral or nasal route, administer according to directions on package insert. 10. Check medication label and verify with order, drawing up appropriate dose (if applicable) 11. Confirm with patient immunization to be given prior to administration. 12. Give medication by route (IM, SQ, Orally, ID) as appropriate – per package insert. 13. Instruct patient or caregiver about expected reaction, possible side effects and if appropriate, time interval between immunization. Information is given regarding the time interval during which reaction is likely to occur, symptoms, action to alleviate symptoms, duration of symptoms, and time interval between immunizations, if the immunization is part of a series. Provide Health Facts For You #5240- Immunizations for Children and Adults. 14. Observe patient for 15 minutes following injection and observe for any untoward reaction. 5 DFM Policy & Procedure Manual □ Clinical Operations □ Faculty Council □ Administrative X□Other Effective Date: 1/1/2011 X□ Original Page __6____ __ 7_ Policy # □Revision Title: Immunization Policy 15. Notify physician immediately in the event of respiratory distress, anaphylaxis or angioneurotic edema (swelling of throat and pharynx) or call a ‘code blue’ if necessary. Refer to UWMF Anaphylactic Protocol for treatment guide. 16. Report all untoward reaction to immunizations, such as fever, rash, abscesses to the physician. 17. Follow the DFM Vaccine Refusal Policy and Procedure, should a patient or responsible party refuse a routinely recommended vaccine for their child, after educating the patient or responsible party of the benefits and risks of the vaccine, and the risks of refusing the vaccine. HealthLink or other type of patient medical record, document the following items: vaccine used date of immunization name of the person giving the immunization publication date of the VIS which was given in Progress Note area type in .npwimm and complete text (HealthLink) signed refusal, if warranted Wisconsin Immunization Registry (WIR): Immunization given Dose given Injection site Route of administration Lot number Manufacturer Expiration date Name of ordering clinician Name of person administering immunization 6 DFM Policy & Procedure Manual □ Clinical Operations □ Faculty Council □ Administrative Effective Date: 1/1/2011 X□ Original Page __7____ __ 7__ Policy # □Revision Title: X□Other Immunization Policy AFTER INJECTION PROCEDURE 1. Before discharge from the clinical area, advise the patient and/or family regarding possible reactions and their treatment. 2. Report any adverse reactions to the provider and record them in the patient’s medical record. Report all significant adverse reactions to the federal Vaccine Adverse Event Reporting System at www.vaers.hhs.gov or (800)-822-7967. 3. Activate a process or system in place to remind and recall those patients who are overdue for recommended immunizations. REFERENCES 1. Center for Disease Control. www.cdc.gov 2. http://www.immunize.org 3. UW Health Immunization Task Force WRITTEN BY: UW Health Immunization Task Force REVISED BY: Sandy Jacobson, Clinic Operations Manager, April 9, 2010 For DFM specific clinics – Sue Kaletka, Director of Clinical Care Services, December 17, 2010 REVIEWED BY: UW Health Immunization Task Force, Chaired by James Conway, M.D, 2010 LaVay Morrison, RN, BSN, Clinical Staff Educator, 2010 Authorization: Date: 12/20/2010 7