Molecular Weight of a Volatile Liquid * 1991 AP Exam Lab Question
advertisement
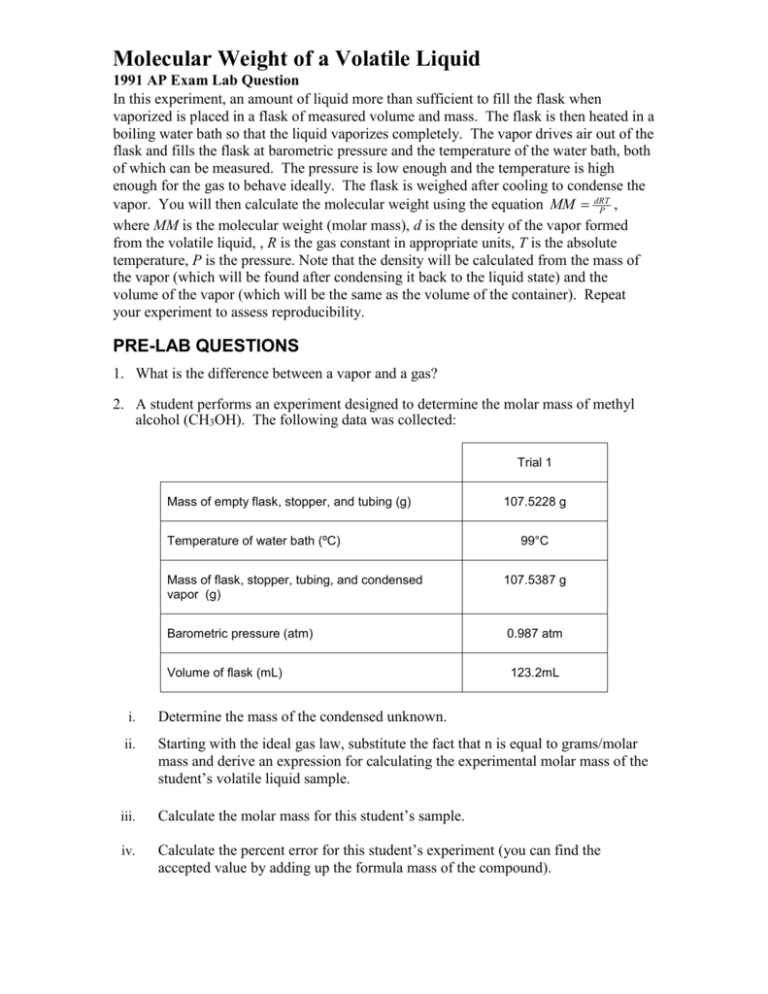
Molecular Weight of a Volatile Liquid 1991 AP Exam Lab Question In this experiment, an amount of liquid more than sufficient to fill the flask when vaporized is placed in a flask of measured volume and mass. The flask is then heated in a boiling water bath so that the liquid vaporizes completely. The vapor drives air out of the flask and fills the flask at barometric pressure and the temperature of the water bath, both of which can be measured. The pressure is low enough and the temperature is high enough for the gas to behave ideally. The flask is weighed after cooling to condense the vapor. You will then calculate the molecular weight using the equation MM dRT , P where MM is the molecular weight (molar mass), d is the density of the vapor formed from the volatile liquid, , R is the gas constant in appropriate units, T is the absolute temperature, P is the pressure. Note that the density will be calculated from the mass of the vapor (which will be found after condensing it back to the liquid state) and the volume of the vapor (which will be the same as the volume of the container). Repeat your experiment to assess reproducibility. PRE-LAB QUESTIONS 1. What is the difference between a vapor and a gas? 2. A student performs an experiment designed to determine the molar mass of methyl alcohol (CH3OH). The following data was collected: Trial 1 Mass of empty flask, stopper, and tubing (g) Temperature of water bath (ºC) 107.5228 g 99°C Mass of flask, stopper, tubing, and condensed vapor (g) 107.5387 g Barometric pressure (atm) 0.987 atm Volume of flask (mL) 123.2mL i. Determine the mass of the condensed unknown. ii. Starting with the ideal gas law, substitute the fact that n is equal to grams/molar mass and derive an expression for calculating the experimental molar mass of the student’s volatile liquid sample. iii. Calculate the molar mass for this student’s sample. iv. Calculate the percent error for this student’s experiment (you can find the accepted value by adding up the formula mass of the compound). Molecular Weight of a Volatile Liquid 1991 AP Exam Lab Question MATERIALS 125 mL flask ring stand volatile organic liquid clamp 600 mL beaker (1000 mL works even better) tongs thermometers barometer analytical balance 250 mL graduated cylinder Red food color One hole stopper to fit flask Glass tubing to fit stopper Glycerin Hot plate PROCEDURE 1. Fill your beaker about two-thirds full of water, add a drop of red food color and begin to heat to boiling. Try to keep the temperature just under 100 oC. 2. Place a piece of glass tubing into a 1-hole rubber stopper. 3. Find the mass of the empty flask with the stopper and tubing. 4. Add 3 mL of an organic liquid to the flask, and stopper it. 5. Clamp the flask to a ringstand and immerse the flask up to its neck in boiling water. Have the flask tilted at a slight angle so you can see when all the liquid has evaporated. The flask should be at least 0.5 cm from the bottom of the beaker. The volatile organic liquid will begin to vaporize immediately. Measure the temperature of the water by placing the bulb of the thermometer on a level with the middle of the flask. Record. 6. Heat until all liquid evaporates and excess vapor is no longer expelled. You will need to heat for at least 1 minute after you perceive that all the liquid has evaporated. You can try to test this by holding a cool watch glass near the top of the glass tubing to see if any vapor will condense. 7. Remove the flask and cool under tap water. Dry the outside of the flask and reweigh the flask with the condensed organic liquid. (The weight of the condensed liquid, and thus the vapor, can be found by subtraction). Discard the liquid. Molecular Weight of a Volatile Liquid 1991 AP Exam Lab Question 8. Record the barometric pressure. This equals the total pressure of gasses in the flask throughout the experiment. Also measure room temperature. 9. Find the volume of the flask. Fill the flask with water (to the top, don’t forget the stopper) then measure the volume of water by pouring it into a graduated cylinder. 10. Calculate the molecular weight of the unknown. DATA TABLE Mass of empty flask,rubber stopper, and tubing Mass of flask, rubber stopper, tubing, and condensed vapor Temperature of water bath Barometric pressure Volume of the flask (mL) POST LAB QUESTIONS AND DATA ANALYSIS 1. Determine the mass of the condensed unknown. 2. Use the expression you derived for Pre-Lab Question # 2 part iii. along with the data collected to calculate the molar mass of your unknown compound. 3. Use your experimentally determined molar mass and reference material to identify the unknown volatile liquid you tested. 4. Obtain the accepted value for your molar mass from your teacher. Calculate your % error. 5. A student failed to vaporize the entire sample prior to cooling the flask under the cool tap water. How did this error affect the calculated molar mass? Justify your answer using calculations. 6. a. Describe a different method you could use to find the volume of the flask that would be more accurate than the method we used. b. A student failed to dry the outside of the flask prior to massing it using the method described in part a. How did this error affect the calculated molar mass? Justify your answer using calculations. 7. How would your calculated molar mass have been affected if you had used twice the initial amount of the unknown compound?






