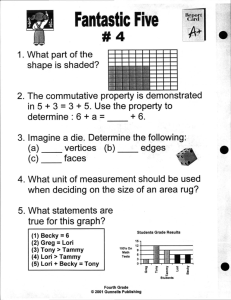
Name: Regents Earth Science Date ______ Period: _____ Lab 9
advertisement

Name: ______________________ Date _______ Regents Earth Science Period: _____ Lab 9: Density of a Solid Question: How can the density of a solid material be determined? Background: 1) Which is heavier – a pound of feather or a pound of lead? __________ Which is more dense, lead or feathers? ________________ Weight and density are often confused. 2) Density is defined as __________________________________________________. In this investigation density will be expressed in grams per cubic centimeter (g/cm3) , The formula for calculating density is: Density = ---------------------- or D = --------- 3) Most Earth materials tend to be most dense in the _______________ state, or phase. Water, however, is most dense as a liquid at 4oC and expands when it freezes, or becomes a solid. Therefore, if the mass of the water remains constant, and the volume expands, the density will ____________________. The solid Earth has an average density which is 3.32 g/cm3. The density of each planet in our solar system varies. For example the density of Jupiter is 1.33g/cm3. Density differences are responsible for many of Earth’s processes, including the weather “machine” and convection currents in the mantle. These convection currents drive the great lithosphere plates in their large-scale movement or plate tectonics. Determining Volume Volume can be determined in one of several ways. For rectangular solids, it can be calculated form direct measurements by multiplying length x width x height. 4) Practice: Measure the block above: Length = _____________ width = _____________ height = _____________ Calculate Volume: _______________ The block above has a mass of 2.5 g. 5) Calculate the block’s density (Show Work: equation, substitution, answer with units). 6) Sample # 1 is aluminum. : mass of your sample: _________________ Volume of your sample: _________________.....YOUR calculated density: _________________ 7) What is the density of aluminum? __________________ 1 8) Using your ESRT, p. 10, which layer of the Earth would aluminum be found in, using density ONLY? Calculating the Density of Irregular Solids: find volume using water displacement . . 9) Sample # 2 is galena : mass of your sample: _________________ Volume of your sample: _________________.....YOUR calculated density: _________________ 10) Using your ESRT, p. 10, which layer of the Earth would galena be found in, using density ONLY? Analysis and Conclusions: 11) What is the difference between the following measurements? 2cm 2cm 2 2cm 3? ________________________________________________________________________________ 12) What were some possible sources of error in your density determination? ________________________________________________________________________________ 2 13) When determining the density of any pure material, does the size or shape of your sample affect the density value that you determine? Explain why, by supporting your answer with evidence from this lab. ________________________________________________________________________________ 14) Make a list of the other reference tables in the ESRT show the density of something? ESRT Table # Density of: 3