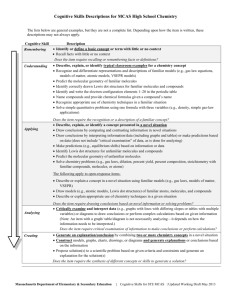
ORGANIC CHEMISTRY CH 3310-01
advertisement

General Chemistry I Fall 2008 CH2130.03 in Boyd 239, MWF 9:05am (Dr. Aparna Waghe) CH2130.02 in Boyd 239, MWF 10:10 am (Dr. Anil Waghe) CH2130.01 in Boyd 239, MWF 11:15 am (Dr. Susan Swope) CH2130.04 in Boyd 239, MWF 12:20 pm (Dr. Aparna Waghe) Instructors: Dr. Susan Swope Office: Boyd 110 Phone: 535-2481 E-mail: sks@plymouth.edu Office hours: MWF 10-11 am; T 8-9 am Dr. Anil Waghe Office: Boyd 102 Phone: 535-3243 E-mail: awaghe@plymouth.edu Office Hours: MWF 9-10am; R 11-12 I. Dr. Aparna Waghe Office: Boyd 118a Phone: 535-3251 E-mail: aawaghe@plymouth.edu Office hours: MTWF 11-12 am Introduction CH 2130 is the first semester of a two-semester sequence in general chemistry. The course sequence CH 2130 and CH 2140 is designed to satisfy the general chemistry requirement for science majors. The laboratory component of the course (CH2230) must be taken concurrently with the course. It is assumed that students enrolled in this course have basic quantitative skills that include algebra. II. Course Objectives Chemistry is the study of matter and the changes it undergoes. Chemistry is known as the central science, not only because it is central to the study of other sciences, but also because it is central to our understanding of the natural world. Everything that we see, touch, smell and feel is matter, and all of our senses transmit information via chemical interactions. In addition, most of the pressing societal issues involve chemistry, and thus our ability to understand and evaluate these issues demands a basic knowledge of the chemical sciences. Consequently, a major aim of this course is to introduce the chemical concepts fundamental to understanding and solving chemical questions. The major concepts addressed in the course are the following: • Attractions between positive and negative centers hold matter together and are responsible for chemical reactions. • The lower its energy, the more stable the system. • During change, energy is conserved: Enet = 0. • The properties of elements repeat periodically as the atomic number of their atoms increases. • Electrons in atoms and molecules act like matter waves with quantized energies; the more spread out a matter wave, the lower (more favorable) its energy. • Change occurs in the direction that increases the number of distinguishable arrangements of particles and/or energy quanta. Entropy, S, is a measure of this number, and in all spontaneous processes, net entropy increases, Snet > 0. • Reactions are at equilibrium when Snet = 0 for the change from reactants to products. • When a reaction at equilibrium is disturbed, the system reacts to minimize the disturbance. This is LeChatelier’s principle. Reactions at equilibrium are quantitatively described by a temperature dependent equilibrium constant ratio. • Electric current can produce reduction-oxidation chemical reactions. Reduction-oxidation chemical reactions can produce an electric current. • The rate of a chemical reaction depends on the concentrations of species and the temperature of the system. These are a result of the reaction pathway. In addition to these “big ideas”, specific learning outcomes for each chapter are available on the Web CT version of the syllabus. Chemistry is an analytical and quantitative science, and students will gain experience solving problems and gaining understanding from the scientific perspective. This includes reasoning by analogy, using models to visualize the atomic and molecular world, practice with problem-solving tools and strategies such as dimensional analysis, graphical analysis, and the use of common sense to solve problems. The laboratory is an essential component of the general chemistry curriculum. Here, students learn some of the basic laboratory skills used to understand the physical and chemical properties of matter. Students learn to read and make sense of laboratory procedures, make careful observations and measurements, and organize, record and analyze data in a laboratory notebook and write a laboratory report. III. Format and Procedures Many of you will find that the format for this course is different from previous science courses. We strongly suggest that you carefully read the Introduction from your text for an explanation of the various activities and assignments that will be part of the course: ‘Investigate This’; ‘Consider This’; ‘Worked Example’; and ‘Check This’. “Chemists and other scientists learn about the world through experimenting. They then try, often in collaborative efforts, to develop models of the world at the molecular level that explain their results and allow them to predict the outcomes of other possible experiments. We have tried to incorporate this same approach in this (class).” (ACS Chemistry text). New topics are introduced in class using informal lectures and Powerpoint presentations. Students are then challenged to apply these concepts to answer questions and solve problems, first in class and then on other homework assignments. In-class worksheets are distributed regularly, and these problems are discussed and often completed in class. Blue book quizzes are given at the beginning of most classes; these are used to encourage good study habits as well as provide feedback for the instructor. Students are expected to attend class regularly to take an active role in the class by participating in in-class problem solving, by asking questions, and by participating in discussions during class. IV. My Assumptions Students should regularly log in to Blackboard and read the assignments prior to attending the class. Students should prepare for class by reading the assigned text material and reviewing the assigned problems. After class, students are expected to work out any assigned problems, individually, or preferably with a study group. Although the amount of study time required to master the material in this course will vary from student to student, students should expect to spend three hours outside of class for every hour in class. This work should be done on a regular basis, not saved until a day or two before the exam. V. Course Requirements 1. Course Materials: a. Required text: Chemistry (A project of the American Chemical Society), Freeman, 2004. b. Molecular modeling kit 2. A scientific calculator is required and should be brought to class every day. Please read and understand how to use various buttons/functions like parentheses, log, ln, ex, 10x, etc. VI. Grading/Assessment Hour Exams (3): Final Exam: Problem Sets: Blue book quizzes: Group "quizzes": 45% 15 % (or 30% and drop one hour exam grade)* 15% 15%* 10% Hour Exam and Final Exam: These are the most comprehensive assessment tools. Exams are designed to evaluate the descriptive, conceptual and problem-solving goals of the course. Three hour-exams and the final exam will be given on the dates indicated in the course schedule. The final exam is cumulative. Blue book Quizzes: These are designed to promote good study habits and to indicate areas of strength and weakness for student and instructor; quizzes assess both conceptual and problem-solving goals. Short blue book quizzes will generally be given twice weekly. Group Quizzes: Group quizzes will involve evaluation of group work on collaborative quizzes, group summary sheets, and/or group results presented to the class. Note that the instructor reserves the right to give no credit for a group member who is not contributing to the group effort. Problem Sets: These are used to test your problem-solving skill and give practice explaining and applying concepts. You should do all the assigned problems either alone, or preferably in study groups, and be sure to pass the completed assignments in on time. All problem sets will be graded and answer keys will be posted on Blackboard. Homework: In addition, suggested additional problems will be assigned to give you the extra practice you may need to effectively synthesize the material from the classroom and the textbook readings. These problems will not be passed in or graded, but variations of many of these problems will appear on subsequent quizzes and hour exams. Note that it is not possible to make up a quiz due to unexcused absence or to get credit for problem sets passed in after the due date. *Students will have the option of double-counting their final exam grade (30%) and dropping their lowest hour exam grade. In addition, the lowest 3 quiz grades will be dropped. VII. Student Support Many students find General Chemistry among their most challenging courses. Students are encouraged to work with each other on assigned problems, in study sessions for quizzes and exams, and on concepts presented in class and assigned readings. Take advantage of faculty office hours, Blackboard mail, and time before and after class and lab to ask questions or schedule individual help sessions. Working with peers is often among the most successful way to improve problem- solving skills and address misconceptions. The Chemistry Resource Center (Boyd 138) is a place where students may go to work with other students. Tutoring sessions are staffed by students who have been successful in General Chemistry and are interested in helping other students learn chemistry. The sessions are held Monday through Thursdays in the late afternoon and early evening. (See Blackboard Discussion for schedule). VIII. Tentative Course Schedule The following is an overview of the topics covered and the hour exam schedule. Detailed reading assignments, assigned problems and quiz schedule will be posted on the Blackboard calendar. Note that although the coverage of topics may be altered slightly over the course of the semester, the hour exam schedule will not change. Date Sept 3 - Sept 24 Topic 1. Water 2. Aqueous Solutions and Solubility Wednesday, Sept 24 Hour Exam 1 Sept 26 - Oct 22 2. Aqueous Solutions and Solubility 3. Origin of Atoms Wednesday, Oct 22 Hour Exam 2 Oct 24 - Nov19 4. Structure of Atoms 5. Structure of Molecules Wednesday, Nov 19 Hour Exam 3 Nov 21 - Dec 12 6. Chemical Reactions December 15-19 Final Exam Chapters from Chemistry Chapter 1 & 2 6:00 pm in Boyd 144 Chapter 2 &3 6:00 pm in Boyd 144 Chapter 4 & 5 6:00 pm in Boyd 144 Chapter 6 Chapters 1-6 in Boyd 239 **See Blackboard calendar for detailed weekly postings on topics and assignments. IX. Blackboard Students registered for the course will have access to Blackboard. Here you will find: 1. a Calendar with your weekly assignments 2. a Discussion section where you will find timely information on tutoring schedules, changes in assignments, and other announcements 3. Course Outlines in Powerpoint, labeled by date presented in class. 4. Answer Keys to all hour exams, quizzes, and in-class worksheets 5. My Grades. Your grades for all assignments are recorded here 6. Web Links. Useful sites on the Web. 7. Web Mail. An easy way to contact the instructor. X. Learning Outcomes (see syllabus on Blackboard) Chapter 1 • describe solids, liquids, and gases in terms of their macroscopic properties and write or draw molecular-level descriptions that explain these properties [Section 1.1]. • make drawings that show how the electrical nature of matter explains the results of electrostatic experiments [Section 1.2]. • use the nuclear atomic model, the shell model for electrons, and the periodic table to determine the charge on the atomic core and the number of valence electrons in an atom [Section 1.2]. • use the periodic table and the atomic shell model to predict trends in atomic size and electronegativities [Sections 1.2 and 1.6]. • describe the relationships among different molecular models and the information that each of them provides [Sections 1.3, 1.4, 1.5, and 1.6]. • write Lewis structures for molecules whose molecular formulas contain only first and second period elements (or analogous molecules of higher period elements) [Sections 1.4 and 1.7]. • use drawings, physical models, and words to describe the geometry of the valence electrons and nuclei for molecules whose molecular formulas contain only first and second period elements (or analogous molecules of higher period elements) [Sections 1.5 and 1.7]. • predict the direction and relative magnitude of bond dipoles and the direction of the resultant molecular dipole, including the effect of nonbonding electrons, for simple molecular structures [Section 1.6]. • use drawings, physical models, and words to describe the origin of intermolecular interactions due to London dispersion forces, dipolar attractions, and hydrogen bonding [Section 1.7]. • use intermolecular attractions to predict and/or explain trends in boiling points and energies of vaporization for a series of compounds whose molecular structures you know or can determine [Sections 1.7 and 1.11]. • use drawings, physical models, and words to describe how the structure of the water molecule is responsible for the densities of solid and liquid water, the temperature dependence of the density of liquid water, and the consequences for life on Earth [Section 1.8]. • describe some of the places where hydrogen bonding occurs in biomolecules and explain how hydrogen bonding is important for the functions of these molecules [Section 1.9]. • describe and use energy diagrams to illustrate the direction of energy transfer from one substance to another when phase changes occur [Section 1.10]. • use the relationship among energy change, temperature change, mass, and specific heat to make quantitative comparisons among substances that gain or lose thermal energy [Sections 1.10, 1.11, and 1.12]. • use the molar mass of a compound, determined from the relative atomic masses of its constituent atoms, to calculate the number of moles in a given mass of the compound and vice versa [Section 1.11]. • use drawings, physical models, and words to describe the molecular basis for the differences in specific heats among different compounds [Section 1.12]. • use drawings, physical models, and words to describe the molecular basis for the differences in viscosities among different liquid compounds and for the dependence of viscosity on temperature [Section 1.14]. Chapter 2 • use an energy diagram to characterize the basic steps in the dissolving process and give a molecular level explanation for the direction of the individual and net energy changes [Sections 2.1, 2.2, and 2.5]. • use molecular models, Lewis structures, and other representations of molecules to show how the three major attractions between like and unlike molecules — hydrogen bonding, polar attractions, and London dispersion forces — affect the solubility of a given molecular solute in water [Section 2.2]. • give a molecular level explanation for the favorable and unfavorable factors that determine the solubility of a given molecular solute or ionic compound [Sections 2.2 and 2.7]. • predict the relative aqueous solubilities of a given set of molecular solutes [Section 2.2]. • show the direction of motion of the molecules and ions in a solution being tested with an electrical conductivity tester [Section 2.3]. • write the chemical formula of any ionic compound, given the charges on the cation and anion and name ionic compounds of common ions, given the chemical formula [Sections 2.4 and 2.13]. • draw an energy diagram for the formation of an ionic crystalline compound from its elemental gas phase atoms and give a molecular level explanation for the direction of the energy changes of the individual steps [Section 2.4]. • make a drawing showing the process of dissolving a polar solute or an ionic compound that shows how water molecules hydrate the dissolved molecules or ions [Sections 2.3 and 2.5]. • draw an energy diagram for the dissolution of an ionic crystalline compound in water and give a molecular level explanation for the direction of the energy changes of the individual steps [Section 2.5]. • use lattice and hydration energies to determine whether a given ionic compound will dissolve exothermically or endothermically in water [Section 2.5]. • predict whether a precipitate will form when two ionic solutions are mixed [Sections 2.6 and 2.7]. • carry out these interconversions: grams moles of a compound, moles (grams) of reactant moles (grams) of product in a stoichiometric reaction, and volume of a solution of known concentration moles (grams) of solute in that volume [Sections 2.8, 2.9, 2.10, and 2.14]. • prepare (give step-by-step instructions for preparing) an aqueous solution of a specified molarity in some solute [Section 2.9]. • determine the limiting reactant in a reaction mixture (solution) and the concentrations of all species formed or remaining in the solution when the reaction is complete [Sections 2.10 and 2.14]. • use conductivity and/or pH data to determine whether a solute undergoes an acid-base reaction with water and, if so, write the equation for the chemical reaction [Sections 2.11, 2.12, 2.13, and 2.14]. • use the concentration of hydronium ion, hydroxide ion, or the pH to tell whether an aqueous solution is acidic or basic [Sections 2.12, 2.13, and 2.14]. • write the equation for the reaction between Brønsted-Lowry acids and bases and identify the Brønsted-Lowry conjugate acid-base pairs in any acid-base reaction [Sections 2.12 and 2.14]. • draw a molecular-level diagram and/or explain in words the reactions occurring in a reacting system (dissolution, precipitation, or acid-base) at equilibrium [Sections 2.6, 2.12, 2.13, and 2.14]. • use Le Chatelier’s principle to predict and/or explain the direction of changes observed in a reaction system disturbed by addition or removal of a reactant or product [Sections 2.14 and 2.16]. Chapter 3 • describe a spectrograph and how it produces the spectrum from its light source [Section 3.1]. • distinguish between continuous and line spectra, identify an element from its line emission spectrum, and determine relative amounts of two elements by the brightness of their emission lines [Section 3.1]. • write the complete symbol and locate the subatomic particles in a diagram of any isotope of an elemental atom or ion [Section 3.2]. • explain cosmic condensation and nuclear fusion events as a function of the temperatures that make them possible [Section 3.3]. • 56 write and balance reactions along the pathway for the formation of elements up to about Fe in the first stars [Sections 3.3 and 3.4]. • write and balance reactions along the pathway for the formation of elements beyond 56Fe in the first stars [Sections 3.3 and 3.4]. • describe the formation of planetary systems and the source of their elementary atoms [Sections 3.3 and 3.7]. • balance nuclear reactions (fusion, fission, and radioactive decay) by supplying the appropriate reactant or product nuclei and/or other particles [Section 3.4]. • use half lives to find the amount of a radioactive nucleus remaining after an elapsed time or to find the time elapsed since decay began [Sections 3.4 and 3.7]. • determine the energy changes for mass-to-energy conversions in nuclear fusion and fission reactions and vice versa [Section 3.5]. • determine nuclear binding energies from mass-to-energy conversion in formation of nuclei from protons and neutrons [Section 3.5]. • use nuclear binding energies to predict whether a nucleus is likely to undergo fusion or fission [Section 3.5]. • give the requirements for a nuclear chain reaction and diagram the differences between controlled and uncontrolled chain reactions [Section 3.5]. • use abundance data to estimate abundances of elements relative to one another in the universe, on Earth, and in organisms [Sections, 3.1, 3.6, and 3.7]. • use nuclear binding energies to explain trends in cosmic elemental abundances as well as deviations from the trends [Section 3.6]. • use the model for planetary formation to explain the structure of the Earth and the abundance of elements in the Earth’s crust [Section 3.7]. • relate cosmic elemental abundances to possible evolution of life [Section 3.7]. • describe how the age of a sample can be determined from the initial and present ratios of two isotopes, at least one of which is radioactive [Section 3.9]. • describe how the history of a sample can be determined from the ratio of two stable isotopes of an element in the sample [Section 3.9]. Chapter 4 • identify and describe periodic patterns in atomic and elemental properties and by extrapolation or interpolation predict values of these properties for elements that have not been measured [Sections 4.1 and 4.10]. • explain the relationship between atomic emission and absorption of light [Section 4.2]. • calculate the wavelength, frequency, or speed of propagation of a wave, given two of the three variables or the information necessary to derive them [Section 4.3]. • describe in pictures and/or words how superimposition of waves produces diffraction patterns when waves pass through a grating [Section 4.3]. • identify, given the characteristics of a source or detector of radiation, where in the electromagnetic spectrum the radiation will be found [Section 4.3]. • calculate the wavelength, frequency, or energy of a photon, given two of the three variables or the information necessary to derive them [Section 4.4]. • show how the results of photoelectric-effect experiments can be explained by Planck’s quantum hypothesis and how the wave model fails [Section 4.4]. • identify and explain whether a phenomenon is a result of the wave or photon (quantum) properties of light [Section 4.4]. • explain how the line emission and absorption of light by atoms requires that the energies of atoms be quantized [Section 4.5]. • use an energy level diagram for an atom and emissions from some known energy level changes to predict the emissions from other energy level changes [Section 4.5]. • calculate the de Broglie wavelength of any particle of known mass and velocity and identify where in the electromagnetic spectrum it will be found [Section 4.6]. • characterize a standing wave in terms of its amplitude, wavelength, and nodal properties [Sections 4.7 and 4.11]. • explain why the uncertainty principle leads to a probability picture of an electron wave in an atom instead of more easily visualized orbits of an electron [Section 4.7]. • describe in pictures and/or words how vibrating objects like strings and guitar bodies are related to the probability model of electron waves (orbitals) in atoms [Sections 4.7 and 4.11]. • calculate or predict the direction of change for the kinetic energy, if the mass or velocity of the object changes [Section 4.8]. • calculate or predict the direction of change for the potential energy of a system if the variables that describe the potential energy (charge and distance) are changed [Section 4.8]. • use pictures, words, and/or equations to explain how the potential and kinetic energies of electron waves prevent atoms from collapsing and determine their size [Sections 4.8 and 4.13]. • explain how the balance of potential and kinetic energies of electron waves together with the exclusion principle lead to a shell structure for atoms [Sections 4.9, 4.10, and 4.11]. • show how the periodic properties of atoms and elements provide evidence for (or against) an electron shell and subshell model of atomic structure [Sections 4.10 and 4.11]. • show how the electron shell model for atoms explains and predicts periodic properties of atoms and elements, including, but not limited to, atomic size, ionization energies, and electronegativities [Section 4.10]. • describe the atomic orbitals derived from the Schrödinger wave equation for principal quantum numbers 1 and 2 [Section 4.11]. • write the electron configurations for atoms of the first 20 elements, using the energy levels for multielectron atoms and their degeneracies and accounting for the exclusion principle and electron-electron repulsion energies [Section 4.11]. • predict, from its electron configuration, the number of unpaired electron spins a ground- state atom has [Section 4.11]. • describe how the electron shell model and the orbitals and energies derived from solutions to the wave equation are complementary and together provide a way to explain all the periodic properties of atoms and elements discussed in the chapter [Section 4.1, 4.9, 4.10, and 4.11]. Chapter 5 • write appropriate Lewis structures for molecules whose atomic composition you know, including multiple Lewis structures representing possible isomers [Sections 5.2, 5.5, 5.8 and 5.9]. • build molecular models based on the connectivities shown in Lewis structures of the molecules [Sections 5.2, 5.5, 5.8 and 5.9]. • for a series of compounds, correlate patterns of chemical behavior with their Lewis structures and molecular models and, based on these patterns, predict the behavior of other compounds [Sections 5.1, 5.2, 5.5, and 5.9]. • use Lewis structures to predict multiple bonding in the molecules of a compound [Section 5.5]. • draw and/or describe the , n, and orbitals for simple molecules containing elemental atoms from periods one, two, and three [Sections 5.3, 5.4, 5.6, and 5.7]. • draw and/or describe the sigma framework of a molecule and predict the shape of the molecule [Sections 5.4 and 5.6]. • use Lewis structures to predict whether a molecule is likely to have delocalized orbitals [Sections 5.7 and 5.8]. • use the delocalized molecular orbital (“sea of electrons”) model of metallic bonding to explain the properties of metals [Section 5.7]. • use bonding models to determine bond order in molecules [Sections 5.7 and 5.13]. • use bonding models to predict and/or explain the relative bond lengths in molecules [Sections 5.4, 5.5, 5.6, and 5.7]. • beginning with any one of the following representations of relatively simple molecules, write, draw, or build all the other representations: condensed (line) formula, Lewis structure, 3-d structure, condensed structure, skeletal structure, molecular model [Sections 5.2, 5.5, and 5.8]. • use appropriate molecular representations to show whether a pair of structures are unrelated to one another, are identical to one another, are structural isomers, or are stereoisomers (cis/trans and/or optical isomers) [Sections 5.1, 5.2, 5.8, and 5.9]. • use appropriate molecular representations to show whether structural isomers and/or stereoisomers (cis/trans and/or optical isomers) are possible for a given molecular formula [Sections 5.2, 5.8, and 5.9]. • identify the functional group(s) present in a molecular structure [Section 5.10]. • use the polarities of functional groups (from electronegativities and bond polarities and/or calculated electron distributions) to predict where molecules will be likely to interact with one another [Sections 5.10 and 5.11]. • use pictures and/or words to describe antibonding orbitals [Section 5.13]. • determine the bond order and magnetic properties of molecules containing electrons in antibonding orbitals [Section 5.13]. Chapter 6 • classify a chemical change as a precipitation, Lewis acid-base, or reduction-oxidation reaction based on known reactants and products, or from experimental observations on the change [Sections 6.2, 6.4, 6.6, 6.7, 6.9, and 6.11]. • 6.2]. predict probable precipitation reactions based on the cation and anion charges [Section • use the stoichiometry of continuous variations studies to determine the formula of a reaction product or the ratio in which reactants react [Section 6.2]. • define and give examples of three classes of Lewis acid-base reactions: Brønsted-Lowry proton transfers, metal ion complexation, and nucleophile-electrophile reactions [Sections 6.3, 6.4, 6.6, and 6.7]. • use the observed pH and stoichiometry of a solution to determine the relative basicity (acidity) of the species in the solution [Section 6.4]. • predict the relative basicities (acidities) of a series of Lewis/Brønsted-Lowry bases (acids) of known structure [Section 6.5]. • use relative basicities (acidities) to predict the predominant species in solutions of Lewis/Brønsted-Lowry bases (acids) [Sections 6.4 and 6.5]. • recognize the formation of a metal ion complex between a Lewis base and a metal ion in solution by observations on mixtures of the reactants (or in competitions between the Lewis base and another reactant for the metal ion) and suggest a structure for the complex [Section 6.6]. • identify the nucleophilic and electrophilic sites in a pair of reactants that react to form a condensation product, including condensation polymers [Section 6.7]. • predict the product of a nucleophile-electrophile reaction, including condensation polymerization, between reactants with the functional groups we have introduced [Sections 6.7 and 6.8]. • use formal charge to explain the rearrangements that some reaction intermediates undergo or to explain the relative stability of different isomeric Lewis structures [Section 6.8]. • identify the molecules or ions that are reduced and oxidized and their respective products in a reduction-oxidation reaction [Sections 6.9, 6.10, and 6.11]. • assign oxidation numbers to all the atoms in a given molecule or ion [Sections 6.9 and 6.11]. • balance a given reduction-oxidation reaction in acidic or basic solution by inspection, the oxidation-number method, or the half-reactions method [Sections 6.9, 6.10, and 6.11]. • use your knowledge of the oxidation numbers of atoms in various molecules or ions to predict possible reduced or oxidized products from a reaction [Sections 6.10 and 6.11]. • use oxidation numbers to show that a given reaction is an internal reduction-oxidation [Section 6.11]. • explain how a titration is carried out and used to determine the amount of a reactant present in a solution of unknown concentration [Section 6.13]. • use titration data to determine the amount of a reactant present in a solution of unknown concentration [Section 6.13]. General Chemistry II – CH 2140 Spring 2009 CH 2140.01 in Boyd 239, MWF 9:05am (Dr. Aparna Waghe) CH 2140.02 in Boyd 239, MWF 10:10 am (Dr. Susan Swope) CH 2140.05 in Boyd 239, MWF 11:15 am (Dr. Anil Waghe) CH 2140.03 in Boyd 239, MWF 12:20 pm (Dr. Aparna Waghe) Instructors: Dr. Susan Swope Office: Boyd 110 Phone: 535-2481 E-mail: sks@plymouth.edu Office hours: M 2-3pm; T 9-10am WF 9-10am Dr. Anil Waghe Office: Boyd 102 Phone: 535-3243 E-mail: awaghe@plymouth.edu Office Hours: MWF 10-11am; R 3-4pm Dr. Aparna Waghe Office: Boyd 118A Phone: 535-3251 E-mail: aawaghe@plymouth.edu Office hours: MWF 11-12, T 10-11am Lab Coordinator/Technician: Mrs. Marguerite Crowell Office: Boyd 118B Phone: 535-2272 E-mail: mcrowell@plymouth.edu I. Introduction CH 2140 is the second semester of a two-semester sequence in general chemistry; General Chemistry I (CH2130), or its equivalent, is a pre-requisite for this course. The course sequence CH 2130 and CH 2140 is designed to satisfy the general chemistry requirement for science majors. The laboratory component of the course (CH2240) must be taken concurrently with the course. It is assumed that students enrolled in this course have basic quantitative skills that include algebra. II. Course Objectives Chemistry is the study of matter and the changes it undergoes. Chemistry is known as the central science, not only because it is central to the study of other sciences, but also because it is central to our understanding of the natural world. Everything that we see, touch, smell and feel is matter, and all of our senses transmit information via chemical interactions. In addition, most of the pressing societal issues involve chemistry, and thus our ability to understand and evaluate these issues demands a basic knowledge of the chemical sciences. Consequently, a major aim of this course is to introduce the chemical concepts fundamental to understanding and solving chemical questions. The major concepts addressed in the course are the following: • Attractions between positive and negative centers hold matter together and are responsible for chemical reactions. • The lower its energy, the more stable the system. • During change, energy is conserved: Enet = 0. • The properties of elements repeat periodically as the atomic number of their atoms increases. • Electrons in atoms and molecules act like matter waves with quantized energies; the more spread out a matter wave, the lower (more favorable) its energy. • Change occurs in the direction that increases the number of distinguishable arrangements of particles and/or energy quanta. Entropy, S, is a measure of this number, and in all spontaneous processes, net entropy increases, Snet > 0. • Reactions are at equilibrium when Snet = 0 for the change from reactants to products. • When a reaction at equilibrium is disturbed, the system reacts to minimize the disturbance. This is LeChatelier’s principle. Reactions at equilibrium are quantitatively described by a temperature dependent equilibrium constant ratio. • Electric current can produce reduction-oxidation chemical reactions. Reduction-oxidation chemical reactions can produce an electric current. • The rate of a chemical reaction depends on the concentrations of species and the temperature of the system. These are a result of the reaction pathway. In addition to these “big ideas, specific learning outcomes for each chapter are available on the BlackBoard version of the syllabus. Chemistry is an analytical and quantitative science, and students will gain experience solving problems and gaining understanding from the scientific perspective. This includes reasoning by analogy, using models to visualize the atomic and molecular world, practice with problem-solving tools and strategies such as dimensional analysis, graphical analysis, and the use of common sense to solve problems. The laboratory is an essential component of the general chemistry curriculum. Here, students learn some of the basic laboratory skills used to understand the physical and chemical properties of matter. Students learn to read and make sense of laboratory procedures, make careful observations and measurements, and organize, record and analyze data in a laboratory notebook and write a laboratory report. III. Format and Procedures Many of you will find that the format for this course is different from previous science courses. We strongly suggest that you carefully read the Introduction from your text for an explanation of the various activities and assignments that will be part of the course: Investigate This; Consider This; Worked Example; and Check This. “Chemists and other scientists learn about the world through experimenting. They then try, often in collaborative efforts, to develop models of the world at the molecular level that explain their results and allow them to predict the outcomes of other possible experiments. We have tried to incorporate this same approach in this (class).” (ACS Chemistry text). New topics are introduced in class using informal lectures and Powerpoint presentations. Students are then challenged to apply these concepts to answer questions and solve problems, first in class and then on other homework assignments. In-class worksheets are distributed regularly, and these problems are discussed and often completed in class. Blue book quizzes are given at the end of most classes; these are used to encourage good study habits as well as provide feedback for the instructor. Students are expected to attend class regularly to take an active role within the structured group setting. IV. My Assumptions Students should regularly log in to BlackBoard and read the assignments prior to attending the class. Students should prepare for class by reading the assigned text material and reviewing the assigned problems. After class, students are expected to work out any assigned problems, individually, or preferably with a study group. Although the amount of study time required to master the material in this course will vary from student to student, students should expect to spend three hours outside of class for every hour in class. This work should be done on a regular basis, not saved until a day or two before the exam. V. Course Requirements 3. Course Materials: a. Required text: Chemistry (A project of the American Chemical Society), Freeman, 2004. b. Molecular modeling kit 4. A scientific calculator is required and should be brought to class. Please read and understand how to use various buttons/functions like parentheses, log, ln, ex, 10x, etc. VI. Grading/Assessment Hour Exams (3): Final Exam: Quizzes: Problem Sets: 45% (lowest of 3 grades may be replaced by final) 20% or 35% (if one hour exam dropped) 20% 15% Hour Exam and Final Exam: These are the most comprehensive assessment tools. Exams are designed to evaluate the descriptive, conceptual and problem-solving goals of the course. Three hour-exams and the final exam will be given on the dates and time indicated in the course schedule. The final exam is cumulative. Students will have the option of dropping their lowest grade in an hour exams, in which case the lowest exam score will be replaced by the final exam score. Quizzes: These are designed to promote good study habits and to indicate areas of strength and weakness for student and instructor; quizzes assess both conceptual and problem-solving goals. Short blue book quizzes will generally be given every Wednesday and Friday. It is not possible to make up a quiz due to absence. The lowest 3 quiz grades will be dropped. Problem Sets: These are used to test your problem-solving skills and give practice explaining and applying concepts. You should do all the assigned problems either alone, or preferably in study groups. Problem set will be assigned, collected and graded. Note that it is not possible to get credit for problem sets passed in after the due date. Homework: Suggested additional problems (homework) will be assigned daily to give you the extra practice you may need to effectively synthesize the material from the classroom and the textbook readings. Homework assignment will not be collected, however some questions from homework with minor alterations will be used for quizzes and/or exams. Academic Integrity: Students are expected to abide by the PSU Code of Academic Integrity. See http://www.plymouth.edu/registrar/policies/academic_standing.html Group participation: Class attendance is mandatory. Successful group dynamics will maximize learning in this class. It is therefore essential for all members of the group to attend class regularly and participate fully on each assignment and activity. VII. Student Support Many students find General Chemistry among their most challenging courses. Students are encouraged to work with each other on assigned problems, in study sessions for quizzes and exams, and on integrative problems. Take advantage of faculty office hours, BlackBoard mail, and time before and after class and lab to ask questions or schedule individual help sessions. Working with peers is often among the most successful way to improve problem- solving skills and address misconceptions. The Chemistry Resource Center (Boyd 138) is a place where students may go to get peer tutoring. The tutoring sessions are staffed by students who have been successful in General Chemistry (most are chemistry majors) and are interested in helping other students learn chemistry. The sessions are held Monday through Thursdays in the late afternoon and early evening. (See BlackBoard for schedule. It is also posted outside Boyd 239 and Boyd 138. VIII. Tentative Course Schedule The following is an overview of the topics covered and the hour exam schedule. Detailed reading assignments, assigned problems and quiz schedule are posted on the BlackBoard calendar. Note that although the coverage of topics may be altered slightly over the course of the semester, the hour exam schedule will not change. Date Jan 26 - Feb 9 Feb 13 - Feb 27 Wed., Feb 18 Topic Chemical Reactions Chemical Energetics I Hour Exam 1 Mar 2 - Mar 23 Wed., Mar 25 Entropy Hour Exam 2 Mar 27 – Apr 8 Apr 10 – Apr 24 Wed., April 22 Chemical Equilibrium Reduction-Oxidation Hour Exam 3 Apr 27 - May 8 Finals Week (May 11-16) Reaction Pathways Final Exam Chapters from Chemistry Chapter 6 Chapter 7 Ch. 6 & 1st half of Ch.7 6:00 pm in Boyd 144 Chapter 8 nd 2 half of Ch 7 & Ch 8 6:00 pm in Boyd 144 Chapter 9 Chapter 10 Chapter 9 & 10 6:00 pm in Boyd 144 Chapter 11 Chapters 6-11 **See BlackBoard calendar for detailed weekly postings on topics and assignments. IX. BlackBoard Students registered for the course will have access to Lecture Combo section of this course on BlackBoard . Here you will find: 8. a Course Content section where you’ll find tutoring schedule, problem sets, answer to problem set etc. 9. a Calendar with your weekly assignments, lecture schedule, exam dates etc. 10. an Announcement section where you will find timely information on any changes in assignments, tutoring schedule, and other announcements. 11. Web Links. Useful sites on the Web. Note: Your grades will be recorded in My Grades in respective lecture section, not in lecture combo section. X. Learning Outcomes (see BlackBoard version of syllabus) Chapter 6 • classify a chemical change as a precipitation, Lewis acid-base, or reduction-oxidation reaction based on known reactants and products, or from experimental observations on the change [Sections 6.2, 6.4, 6.6, 6.7, 6.9, and 6.11]. • predict probable precipitation reactions based on the cation and anion charges [Section 6.2]. • use the stoichiometry of continuous variations studies to determine the formula of a reaction product or the ratio in which reactants react [Section 6.2]. • define and give examples of three classes of Lewis acid-base reactions: Brønsted-Lowry proton transfers, metal ion complexation, and nucleophile-electrophile reactions [Sections 6.3, 6.4, 6.6, and 6.7]. • use the observed pH and stoichiometry of a solution to determine the relative basicity (acidity) of the species in the solution [Section 6.4]. • predict the relative basicities (acidities) of a series of Lewis/Brønsted-Lowry bases (acids) of known structure [Section 6.5]. • use relative basicities (acidities) to predict the predominant species in solutions of Lewis/Brønsted-Lowry bases (acids) [Sections 6.4 and 6.5]. • recognize the formation of a metal ion complex between a Lewis base and a metal ion in solution by observations on mixtures of the reactants (or in competitions between the Lewis base and another reactant for the metal ion) and suggest a structure for the complex [Section 6.6]. • identify the nucleophilic and electrophilic sites in a pair of reactants that react to form a condensation product, including condensation polymers [Section 6.7]. • predict the product of a nucleophile-electrophile reaction, including condensation polymerization, between reactants with the functional groups we have introduced [Sections 6.7 and 6.8]. • use formal charge to explain the rearrangements that some reaction intermediates undergo or to explain the relative stability of different isomeric Lewis structures [Section 6.8]. • identify the molecules or ions that are reduced and oxidized and their respective products in a reduction-oxidation reaction [Sections 6.9, 6.10, and 6.11]. • assign oxidation numbers to all the atoms in a given molecule or ion [Sections 6.9 and 6.11]. • balance a given reduction-oxidation reaction in acidic or basic solution by inspection, the oxidation-number method, or the half-reactions method [Sections 6.9, 6.10, and 6.11]. • use your knowledge of the oxidation numbers of atoms in various molecules or ions to predict possible reduced or oxidized products from a reaction [Sections 6.10 and 6.11]. • use oxidation numbers to show that a given reaction is an internal reduction-oxidation [Section 6.11]. • explain how a titration is carried out and used to determine the amount of a reactant present in a solution of unknown concentration [Section 6.13]. • use titration data to determine the amount of a reactant present in a solution of unknown concentration [Section 6.13]. Chapter 7 • identify the forms of energy transferred in physical and chemical changes and show how energy is conserved in the changes [Sections 7.1, 7.2, 7.3, 7.4, and 7.10]. • draw molecular level representations of thermal energy (undirected kinetic energy) and mechanical energy (directed kinetic energy) transfers [Sections 7.2, 7.3, and 7.10]. • identify whether a thermal energy transfer occurs by radiation, conduction, and/or convection [Section 7.3]. • define and identify the variables that are functions of state and those that are functions of the path for a given change [Sections 7.4 and 7.10]. • identify the system and the relevant surroundings for a given change [Section 7.5]. • define and identify open, closed, and isolated systems [Section 7.5]. • use the data from calorimetric measurements to calculate the thermal energy transferred to or from a reacting system [Section 7.6]. • use the data from constant pressure calorimetric measurements to calculate the enthalpy change for the reacting system [Section 7.6]. • define and give examples of homolytic bond cleavage reactions [Section 7.7]. • write equations for homolytic bond cleavage and homolytic bond formation for compounds, use bond enthalpies to calculate the enthalpy changes associated with these processes, and obtain the enthalpy change for gas phase reactions [Section 7.7]. • draw enthalpy level diagrams that show how bond enthalpies combine to give the enthalpy change for a reaction [Section 7.7]. • use bond enthalpies to predict whether, for given atoms, reactions will favor singly- bonded or multiply-bonded products [Section 7.7]. • define standard states for elements and compounds and write the equations whose enthalpy changes are the standard enthalpies of formation of the compounds [Section 7.8]. • use standard enthalpies of formation to calculate the standard enthalpy change for a reaction [Section 7.8]. • draw enthalpy level diagrams that show how standard enthalpies of formation combine to give the standard enthalpy change for a reaction [Section 7.8]. • define coupled reactions and identify examples based on the definition [Section 7.9]. • show whether coupling between two reactions would be an energetically favorable combination [Section 7.9]. • state the first law of thermodynamics in terms of internal energy, heat, and work and use it to analyze a change that occurs by different pathways [Section 7.10]. • explain the difference between E and qV and between H and qP [Section 7.10]. • calculate E, given H and appropriate pressure and volume or P-V work data for a reaction, and vice versa [Section 7.10]. • determine whether a process is consistent with (allowed) by the first law of thermodynamics [Section 7.11]. • use the kinetic-molecular model of gases to explain the observed effects of changes in P, V, n, or T [Section 7.13]. • calculate the final value for P, V, n, or T, given their initial values and the changes in three of the variables [Section 7.13]. • use the ideal gas equation to calculate the P-V work done on or by a chemical reaction system [Section 7.13]. Chapter 8 • relate the relative probability of two outcomes to the number of ways each outcome can be achieved [Sections 8.2, 8.3, and 8.4]. • calculate the number of distinguishable arrangements of a small number of identical particles (molecules) among a limited number of distinguishable locations [Section 8.3]. • relate mixing to osmosis and be able to explain both in terms of increasing number of distinguishable molecular arrangements [Sections 8.1 and 8.4]. • describe the conditions required for osmosis, predict the direction of osmosis, and calculate the osmotic pressure for aqueous systems [Sections 8.1, 8.4, and 8.13]. • calculate the number of distinguishable arrangements of a small number of identical energy quanta among a limited number of distinguishable atoms [Section 8.5]. • predict the direction and final outcome of energy transfer between two systems containing a countable number of energy quanta distributed among a countable number of distinguishable atoms [Section 8.5]. • relate the results for countable systems, both matter and energy distributions, to real systems [Sections 8.4 and 8.5]. • use the definition of entropy in terms of distinguishable arrangements to predict the relative entropies of different systems, for example, phases of matter or reactants and products of a reaction [Sections 8.7, 8.8, 8.9, 8.10, 8.11, and 8.12]. • distinguish between positional and thermal entropy changes for a process and combine them to determine net entropy change for the process [Sections 8.6, 8.7, 8.8, 8.9, 8.10, 8.11, and 8.12]. • use positional entropy changes and the relative thermal entropy changes for the same energy change at different temperatures to analyze and predict the direction of phase changes in pure compounds and solutions [Sections 8.7 and 8.12]. • state the criterion for equilibrium in chemical systems and relate the state of equilibrium to the positional and thermal entropy changes occurring [Sections 8.7 and 8.8]. • explain the relationship of Gibbs free energy change to net entropy change for a process and use the sign and/or magnitude of either one to predict whether the process is possible, not possible, or in equilibrium [Sections 8.8 and 8.9]. • calculate the free energy change for a process in a system using values for the enthalpy and entropy changes for the process [Sections 8.8 and 8.9]. • use standard enthalpies and standard free energies of formation, and standard entropies (from tabulated values) to calculate the standard enthalpy, free energy, and entropy changes for a reaction [Section 8.9]. • explain in words, equations, diagrams, and/or molecular-level sketches the origin and direction of positional and thermal entropy changes for dissolving ionic and molecular solutes in water and predict the observable outcomes [Sections 8.9 and 8.10]. • explain in words, equations, diagrams, and/or molecular-level sketches the origin and direction of positional and thermal entropy changes for formation of micelles and phospholipid bilayer membranes by ambiphilic molecules [Section 8.11]. • use words and/or molecular level sketches to describe the structure and properties of micelles, bilayer membranes, and liposomes [Section 8.11]. • calculate freezing point lowering, boiling point elevation, and osmotic pressure for solutions [Sections 8.12 and 8.13]. • use experimental values for colligative properties to determine the concentration of solutes in the solution and/or the molar mass of the solute [Sections 8.12 and 8.13]. • connect the formation of organized collections of molecules to increases in positional and/or thermal entropy in the system and surroundings that drive the organization [Sections 8.7, 8.10, 8.11, and 8.14]. • predict the direction of the positional entropy change(s) that must occur to produce the observed effects of heating or cooling a system, such as a rubber band [Section 8.16] Chapter 9 • use Le Chatelier’s principle to predict the direction of the response of an equilibrium system to changes in the concentration(s) of reactants or products [Sections 9.1, 9.3, 9.4, 9.5, 9.6, and 9.9]. • use Le Chatelier’s principle and the response of an equilibrium system to heating or cooling to tell whether the reaction is endothermic or exothermic [Sections 9.1 and 9.8]. • explain how Le Chatelier’s principle is a consequence of the dynamic nature of chemical equilibrium [Section 9.1]. • write the equilibrium constant expression using appropriate concentration ratios, hence defining the equilibrium constant, K, for a balanced chemical reaction [Sections 9.2, 9.3, 9.6, 9.7, and 9.9]. • find Ka and pKa for a weak acid (or Kb and pKb for a weak base) when you have the initial concentrations in the solution and the pH or pOH at equilibrium [Sections 9.3 and 9.4]. • find the pH and/or pOH of a solution of a weak acid or its conjugate base when you have the initial concentrations in the solution and the Ka or pKa for the acid [Sections 9.3 and 9.4]. • find the concentrations of a weak acid and its conjugate base in a solution when you have the initial concentrations in the solution and the Ka or pKa for the acid [Sections 9.3, 9.4, and 9.5]. • find the pH of a buffer solution when you have the initial concentrations of the weak acid and its conjugate base in the solution and the Ka or pKa for the acid [Section 9.4]. • tell how to prepare a buffer solution of a specified pH and specified total concentration of an appropriately selected weak acid-base conjugate pair [Section 9.4]. • predict the direction of change of the net charge on a protein and its consequent behavior in electrophoresis if one amino acid is substituted for another in its structure [Section 9.5]. • find the solubility product, Ksp and pKsp, for an ionic compound when you have data for the solubility of the compound and vice versa [Sections 9.6 and 9.8]. • find the solubility of an ionic compound of known Ksp or pKsp in a solution containing a stoichiometric excess of one of the ions [Section 9.6]. • find Goreaction for a reaction when you have the equilibrium constant, K, for the reaction [Section 9.7, 9.8, and 9.9]. • find the equilibrium constant, K, for a reaction when you have Gof for the reactants and products [Sections 9.7]. • find Horeaction, Goreaction, and Soreaction for a reaction when you have calorimetric data for the reaction and its equilibrium constant, K [Section 9.7]. • find Horeaction, Soreaction, and Goreaction for a reaction when you have values of K for the reaction at two or more temperatures [Section 9.8]. • find Goreaction and K for a reaction at a temperature T when you have Horeaction and Soreaction for the reaction [Section 9.8]. • use the reaction quotient, Q, to find the free energy change, Greaction, for a reaction that is not at equilibrium when you have the standard free energy change, Goreaction, or K for the reaction and the concentrations in the non-equilibrium system [Sections 9.9 and 9.11]. • find Goreaction or Greaction for a coupled reaction when you have Goreaction or Greaction for the individual reactions that are coupled [Section 9.9]. • calculate the entropy of gases at non-standard pressures [Section 9.11]. • show how the entropy of gases at non-standard pressures is related to the free energy and reaction quotient for a gas-phase reaction [Section 9.11]. Chapter 10 • use evidence from experimental observations to write probable half reactions for the reductions and oxidations taking place in an electrolytic cell [Section 10.1]. • describe and draw molecular level diagrams of the processes going on and the flow of charge in an electrochemical cell [Sections 10.1 and 10.2]. • use the Faraday and relationships among time, electric current, cell potential, and cell reaction stoichiometry to calculate the amounts of products from electrolysis or the amount of work available from the reactants in a galvanic cell [Sections 10.1 and 10.5]. • show how to connect two metal-metal ion half cells to make a galvanic cell and explain the role of each component of the cell [Section 10.2]. • use the known cell reaction to identify the anode and cathode of an electrochemical cell [Sections 10.1, 10.2, 10.3, 10.4, and 10.5]. • translate a physical cell set up to the conventional line notation for cells and vice versa [Section 10.2]. • use the known sign for a galvanic cell potential to identify the direction of the cell reaction and the anode and cathode of the cell [Sections 10.3 and 10.4]. • determine an unknown cell potential for a cell reaction by combining cell reactions with known cell potentials to give the desired cell reaction and its cell potential [Sections 10.4, 10.5, 10.6, 10.7, and 10.8]. • use a table of standard reduction potentials to predict the direction of any redox reaction (for which data are given) and determine its standard cell potential [Sections 10.4, 10.5, 10.6, 10.7, and 10.8]. • describe how an electrode senses the redox half reaction in a half cell in which the reduced and oxidized species are both present as dissolved ions and/or molecules [Section 10.6]. • determine free energy change for a cell reaction from cell potential and vice versa [Sections 10.5, 10.6, 10.7, and 10.8]. • apply Le Chatelier’s principle to predict the direction of change of cell potentials as concentrations in the half cells are changed [Sections 10.6, 10.7, 10.8, and 10.10]. • use the Nernst equation, which relates the cell potential, the standard cell potential, and the reaction quotient for the cell reaction, to determine any one of these quantities, if the other two are known [Sections 10.6, 10.7, and 10.8]. • use the standard cell potential to determine the equilibrium constant for a cell reaction and vice versa [Sections 10.6 and 10.8]. • use the Nernst equation to convert standard reduction potentials to reduction potentials under non-standard conditions and vice versa [Sections 10.6, 10.7, and 10.8]. • use a table of standard reduction potentials at pH 7 to predict the direction and standard cell potential at pH 7 for redox reactions in biological systems [Sections 10.7 and 10.8]. • combine free energy changes for redox reactions, complexation equilibria, and solubility equilibria to get the free energy changes and reduction potentials for the net redox reactions that occur in systems with such interrelated reactions [Sections 10.8 and 10.10]. • convert a stepwise series of reactions to a diagram that shows the coupling of reactions and vice versa [Section 10.8]. • use standard reduction potentials to determine the probable sequence of a series of coupled redox reactions and vice versa [Section 10.8]. Chapter 11 • predict the usual direction of the effects of changing concentration, changing temperature, or presence of catalysts on the rate of a reaction [Section 11.1]. • determine the initial rate of a reaction in units of M·s–1 from data for the concentration (or a property that is directly proportional to concentration, such as gas pressure or absorbance) of a reactant or product as a function of time [Section 11.2]. • use initial rate and concentration data to determine the order of a reaction with respect to the concentrations of species in the solution and write the rate law for the reaction [Section 11.3]. • explain in words and/or with drawings how the rate of a reaction depends only on the rate of the rate-limiting step in the reaction pathway [Section 11.4]. • derive the rate law for a reaction whose pathway (mechanism), including knowledge of the rate-limiting step, you are given [Sections 11.4 and 11.5]. • propose a pathway (mechanism) for a reaction that is similar or analogous to one whose pathway you know [Sections 11.4 and 11.5]. • use data for concentration (or a property that is directly proportional to concentration) of a reactant or product as a function of time for a first order reaction to determine the rate constant and half life for the reaction [Section 11.5]. • use rate constants and/or half lives for first order reactions to determine how long a sample has been reacting, given some known initial amount or the amount remaining after a known time [Section 11.5]. • explain what is meant by flooding a reaction and use rate and concentration data for a reaction that is flooded with respect to a species to find the order of reaction with respect to the concentration of that species and/or find out whether the disappearance of another species is first order [Section 11.5]. • use the temperature variation of the rate constant (or variables directly proportional to the rate constant, such as rates with the same concentrations of all species) to determine the activation energy for a reaction [Section 11.6]. • use rate constant and activation energy values to determine the Arrhenius frequency factor for a reaction [Section 11.6]. • construct and interpret an activation energy diagram, given information about the activation energy and enthalpy change for the reaction [Section 11.6]. • describe how encounters between reactants in solution differ from collisions between reactants in the gas phase [Section 11.6]. • describe the origin of the temperature dependence of the rate constant and why the dependence is so strong when the average energy of molecular encounters does not increase so rapidly [Section 11.6]. • explain how light can initiate reactions that would not otherwise occur at low (room) temperature [Section 11.7]. • describe how competing photochemical and thermal reactions can lead to a steady state concentration of a reactive species in a system [Section 11.7]. • explain why some reactions that are highly favored thermodynamically are not observed to occur [Section 11.8]. • describe factors that can be changed to provide favorable kinetics for spontaneous reactions that are not otherwise observable [Section 11.8]. • relate the temperature dependence of an equilibrium constant to the temperature dependences of the forward and reverse rate constants for the reaction [Section 11.8]. • describe the Michaelis-Menten pathway for enzyme-catalyzed reactions and use kinetic data to characterize these reactions, especially their behavior as a function of substrate and enzyme concentrations [Section 11.10]. • describe enzyme specificity in terms of functional and substrate specificity and relate specificity to the characteristics of the active sites of enzymes [Section 11.10]. ORGANIC CHEMISTRY CH 3310-01 Fall 2008 Instructor: Dr. ANIL B. WAGHE Location: Boyd Science Center 001 Telephone: 603-535-3243 Email: awaghe@plymouth.edu Meeting Time: MWF 8:00- 8:50 am (Please be considerate of your colleagues, avoid distractions when class is in progress. Set your alarm clocks and be there before 8:00am.) Office Hours : MWF (9:10-10:00am) and R(11:15am to 12:05 pm) and also by appointment at Boyd 102. (I have open door policy so if you see door open you can drop by and ask questions. The office hours and appointment time is the time when I will be in my office just for you.) Text: Required: ORGANIC CHEMISTRY by Janice G. Smith, 2nd Ed. McGraw-Hill Co. 2008 ISBN 978-00-7332749-5 Recommended: STUDENT STUDY GUIDE and SOLUTIONS MANUAL by Janice G. Smith, S. 2nd Ed. McGraw-Hill Co. 2008 ISBN 978-0-07-304987-8 Introduction: Prerequisites for the Organic Chemistry course are both General Chemistry I and II (CH2130 and CH2140) and Co-requisite is CH3330 which is Organic Chemistry Lab. Concepts from general chemistry class will be often used in this course especially in first 3 chapters so please revise them. Earth is the only known planet with life. Since all life on earth involves organic molecules, Organic Chemistry can be considered as a foundation science of life. Studying the interactions and transformations of organic molecules helps us to understand biology and medicine. Organic chemistry has provided us new tools to develop medicine, therapies, plastics, dyes, paints, elastomers, flavors, perfumes and variety of synthetic materials. Organic Chemistry is used in following industries medical & pharmaceutical, chemicals, soap & detergents, paints & pigments, polymers, food & flavors, perfumes, electronics, electroplating, arms & explosives, forensic, environmental, biochemical, cloths, refrigerants, petrochemical … Organic Chemistry Course will be divided into two sections. The first section will be covered during Fall and second section will be covered in Spring. The fall section will be divided in 4 parts as shown in Course Material. Course Objective: Organic Chemistry is the chemistry of compounds that contains the element carbon. Organic Chemistry has lot of information but underneath this vast ocean of information there is a set of principles and an intrinsic logic that is stunningly beautiful. The objective of this course is to comprehend these principles and logic. To appreciate the way organic molecules behave and interact. Study their three dimensional structures and geometries using bonds, electrons, charges. Knowing the classification and nomenclature of organic compounds as well as knowing their functional groups makes it simple to remember properties of huge number of organic compounds. To reach these objectives course has been divided in four subsections for fall. Students will be evaluated with regular quizzes and exams during this period. The labs are coordinated with lecture and they will cover various techniques. Students will be prepared so they can complete a project in spring semester. Course Material: Part I This part will review some physical properties and concepts from general chemistry and give an introduction to Organic chemistry. Properties of molecules and their correlations with electronic structure is major focus of first two chapters. While chapter 3 & 4 will introduce to organic molecules and importance of functional group Chapter 1. Structure and Bonding Chapter 2. Acids and Bases. Chapter 3. Introductions to Organic Molecules and Functional Groups. Chapter 4. Alkanes ----------------------------Exam 1-------------------------------Part II This part will show alkane stereochemistry and overview of simple organic reactions. Chapter 5. Stereochemistry. Chapter 6. Understanding Organic Reactions. ----------------------------Exam 2-------------------------------Part III Saturated compounds (no double or triple bonds) and their reactions. Nomenclature of these compounds and their properties is one of the focuses here. Chapter 7. Alkyl Halides and Nucleophillic Substitution. Chapter 8. Alkyl Halides and Elimination Reactions. Chapter 9. Alcohols, Ethers, and Epoxides. ----------------------------Exam 3-------------------------------Part IV Unsaturated compounds and oxidation, reduction. Chemical reactions and properties of hydrocarbons is the focus here Chapter 10. Alkenes. Chapter 11. Alkynes. Chapter 12. Oxidation and Reduction. ----------------------------Exam 4-------------------------------Please refer WEB-CT for schedule of exams and detailed assignments. I may put PowerPoint presentations, class notes and supporting material on WebCT during the semester. Your success in course will be direct reflection of the work you put in for this course. All the students in class are mature adults. It is very unlikely that you will do well this course, if you frequently skip classes. GRADING/Assessment: There will a 4 exams on each part indicated above. Use of Cell phone or other electronic device will not be allowed during any exam or quizzes. If you expect urgent call or other emergency then you may leave cell phone with the proctor and call will be accepted. Each will worth 100 points Exam 1 = 100 points Exam 2 = 100 points Exam 3 = 100 points Exam 4 = 100 points Final Exam = 100 points. Homeworks + in-class-quizzes = 100 points One LOWEST score from the above 6 categories will be dropped and sum of remaining scores (from 500) will be used to calculate final grade. If there are any changes updates then they will be posted on webCT and announced in the class. Quizzes will be given on every Friday. Make up quiz/home works may not be given if you skip class you lose points under any circumstances. Tentative Exam Schedule: Exam 1 Exam 2 Exam 3 Exam 4 Final Exam Sept 26, 2008 8.00am-8.50am Boyd 001 Oct 10, 2008 8.00am-8.50am Boyd 001 Oct 31, 2008 8.00am-8.50am Boyd 001 Nov 14, 2008 8.00am-8.50am Boyd 001 Dec 15, 2008 8.00 am-11.00am Boyd 001 Student Support: Organic Chemistry is one of the most challenging courses. Students are encouraged to work together however they must present their own work. (DO NOT submit other peoples work as your own without proper citation. Please refer University Plagiarism policy) Take advantage of my office hours and help sessions. I have open door policy if you see me in office I will be available for you. If you need me at certain time you can fix an appointment with me. I sincerely want every one in my class to get best training. I will also arrange Tutoring sessions as semester goes on some experienced students will help you during this semester. Schedules are posted on webCT. Use your solution manual as a guide when you encounter a difficult problem. Please do not copy entire homework from it. Students who did that got significantly lower score in exam than other students. Procedures: Students are expected to attend classes regularly and take active part in class activities. Always read ahead and revise the material on the same day when it is taught. This will significantly help you retain and understand course material. Organic chemistry is a volatile subject. You will understand or you may think you know material during the class but it will be hard to recall after few days. Do not fall behind. It will be very difficult to catch up with all the material just before the exam. Always see me if you need help. Crude Power point presentations are on webCT, however in class presentations will have more information. Use webCT presentations as guidelines and take notes over them to save your time. Multiple choice questions are posted on WebCT. Please practice them after every class. Some of these questions may appear in your exams and quizzes. This is one of the best ways to practice for exam/quizzes. You are encouraged to work in groups but ANY WORK SUBMITTED FOR GRADING should be YOUR ORIGINAL work. If you are not sure ask me before you submit you work. Anyone found cheating in any way will reported to academic integration committee for further actions this may even lead to expelling such student from this institute. Finally Organic Chemistry is one of the enjoyable classes when organize and dedicate few hours per week of your time for it. Organic Chemistry II CH 3320.01 Spring 2009 Syllabus Instructor: Anil Waghe Atmospheric Science and Chemistry Office: Boyd Science Center 102 Phone: 535-3243 Email: awaghe@plymouth.edu Office Hours: MWTF 10:00am-11:00am or by appointment Course Description: This is a 3.0 credit course and this course has PREREQ CH3310 and COREQ CH3340. Course will cover modern identification and structure determination techniques. It will cover properties, nomenclatures, synthesis and reactions of conjugated compounds, aromatic compounds and carbonyl group chemistry. Course Duration: Jan 26, 2009 to May 15, 2009 Class Meeting Time: MWF 8:00-8:50 am in Boyd Science Center 001 Course Objective: After completion of this course you should be able to 1. Identify unknown organic compounds using spectroscopic techniques. 2. Name organic compounds from the structure or draw structures using name. 3. Predict the physical properties of organic compounds from their structures. 4. Design simple synthesis schemes to make organic compounds 5. Explain the major products formed in a reaction. 6. Make proper choice of reagents, solvents and reaction conditions. Required Text: Organic Chemistry 2nd Edition by Janice G. Smith, McGrawHill publisher 2008 ISBN 978-0-0-7332-749-5. Four hour exams will cover 4 parts below Part 1: Spectroscopy (Exam 1: Feb 9, 2009 Monday) Chap 13. Mass Spectrometry and Infrared Spectrometry Chap 14. Nuclear Magnetic Resonance Spectroscopy Part 2: Conjugated compounds (Exam 2: Mar 6, 2009 Friday) Chap 15. Radical Reactions Chap 16. Conjugation, Resonance and Dienes Chap 17. Benzene and Aromatic compounds Part 3: Reactions (Exam 3: Mar 30, 2009 Monday) Chap 18. Electrophilic Aromatic Substitution Chap 19. Carboxylic Acid and acidity of OH Bond Part 4. Carbonyl Chemistry (Exam 4: May 7, 2009 Friday) Chap 20. Organometallic reagents Chap 21. Aldehydes and Ketones Chap 22. Carboxylic acid derivatives Chap 23. Substitution reactions of carbonyl FINAL EXAM Monday May 11, 2009 8:00 am to 10:30 am in Boyd 001 My Expectations: I consider all my students are responsible adults. I expect every student to attend all classes and come prepared for classes. I expect professional conduct and active class participation from my students. Class will begin at 8:00 am and I expect everyone should come few minutes before class start and avoid distractions. Changes and updates will be announced in class and posted on webCT and it is your responsibility to visit webCT and talk to me if you are late/absent for any class. Finally I have not seen ANY student who survives the class after skipped lectures. Do not fall behind and plays catch game. It is extremely difficult in this course. Bring all your problems to me and make sure you are on track all the time. Please refer to university academic integration policy. All the work submitted for grading should be student’s original work with proper citations. Students are encouraged to work in groups but submitted work should NOT be copied from each other, solution manuals or other online sources. Course Assessment: Course assessment will be based on weekly quizzes, homework, exams Exams 100 points each 400 Final 100 points 100 (comprehensive) Homework 10 points each Quizzes 10 points each 100 (H+Q converted to 100) Lowest score from an exam or (H+Q) will be dropped. Total score out remaining 500 will be used to calculate final grade. Exams: You must not use any electronic device Cell phone, camera, mp3, laptop, programmable calculator without prior permission from instructor. Any student found using these devices will be considered as cheating and reported for further action. Final exam will be comprehensive. Quizzes: These are short 10 min quizzes covering material covered during that week. I will have 3-6 short questions. Quiz must be taken during the class and no make-up quiz will be given for unexcused absences. Written note from doctor/coach is required for excused absence. Homeworks: Homework will be 10-16 questions from the end of the chapter. All homeworks will be due on the Wednesday after the chapter is finished. However if there is an exam during the week then the homework will be due on the day of exam. Homework submitted within a week after the due date will get 50% of their grade. Home works submitted after the exam which covers that chapter will get zero points. IF YOU ARE FOUND COPYING HOMEWORK from other student or from solution manual then you will be reported for cheating. Please see me if you need help with homework or classwork. PHYSICAL CHEMISTRY- I (FALL 2008) CH 3450-01 Instructor: Dr. Aparna Waghe Office: Boyd 118A Phone: 603-535-3251 Email: aawaghe@mail.plymouth.edu Office Hours: MTWF 11:00am - noon or by appointment Class Time & Room: MWF 1:25pm – 2:15 pm, Boyd 239 Lab Time & Room: Thursday 9:30am – 12:15pm, Boyd 209 Pre-requisites: CH 2130, CH 2140, MA 2550 & MA 2560 Course Description: Physical chemistry I (CH 3450) is a four credit course with a lab component (see lab syllabus). The main focus of this course is to understand the bulk properties of matter. The emphasis will be on the thermodynamics, solutions, electrochemistry, and chemical kinetics. In brief, the students in this course will study the properties of ideal gas, how do these properties differ for real gases and be able to construct an equation of state that describes these properties. develop the concepts of physical and chemical change in terms of thermodynamics. be able to apply the knowledge of thermodynamics, in particular enthalpy and entropy to make predictions for chemical systems. develop the concepts of chemical potential and how to use it to account for the equilibrium composition of chemical reactions. explore the reaction rates by considering the motion of molecules. study rate laws; be able to construct a rate law from proposed mechanisms. be able to use critical thinking and problem solving skills to solve chemical problems. use calculus, computer spreadsheets and graphics for problem solving Course Text: Required: Atkins’ Physical Chemistry by Peter Atkins and Julio de Paula, 8th Edition (W. H. Freeman & Co., New York, 2006) Recommended: Physical Chemistry - student solution manual by P. Atkins Web site: The web site that accompanies the textbook is available at www.whfreeman.com/pchem8 It offers links to many useful websites, interactive tools such as calculators, plotters, periodic table etc. Calculator: You should bring a simple scientific calculator to every class and exams. No sharing of calculators allowed during exams and quizzes. Course Format: The material will be presented in a lecture format. Powerpoint presentation will be used to discuss various figures, graphs, data etc. Problem solving is an essential part of physical chemistry course. Discussion/question and problem solving will be part every class. All assignments, announcements and grades will be posted on Blackboard, so be sure to check it regularly. Attendance: Regular class attendance and active participation in class discussions, questions and problem solving is expected. It is your responsibility to be aware of all announcements made during the class. Reading: You must read the assigned chapter of the text book before coming to the class, as well as after the material is taught. Course Grade: All students learn at different pace. My goal as an instructor is to encourage good study habits and help you learn. You will have various opportunities to demonstrate your understanding of the course material. Course assessment will be done as follows: Three exams: 15%* each (drop lowest scoring exam) Final Exam: 20% Quizzes: 15% Problem Set: 10% Lab: 25% A ten point scale will be used to assign letter grades, i.e. 90-100% = A, 80-89% = B, etc. Borderline cases will be evaluated by the instructor. Exams: These are designed to assess the understanding of the concepts learned in the course and ability to apply them to solve chemical problems. There will be three exams during the semester. These will be held on the dates given in the class schedule. The lowest scoring exam of the three will be dropped from the final course grade. Final exam will be cumulative and the grade will not be dropped. The date and time of final exam will be as per the university exam schedule. No make-up exams will be given, unless you have a legitimate reason for missing the exam. You need to bring a documentation verifying reason for missing an exam (e.g. doctor’s note). If you cannot take a test, you must contact instructor as soon as possible. You will score zero for any missed exams due to unexcused absence. Homework: End of the chapter problems will be assigned daily for homework. These are assigned to help you understand the material taught in class effectively. The homework will not be graded. However, these problems may be used for quizzes and exams with some modifications. Working in study groups is encouraged but try to attempt problems on your own first. Quizzes: These will be given twice a week to encourage regular study habits. Quizzes will also help assess the strengths and weaknesses of students and instructor in any areas. You are expected to do assigned reading and work out the assigned problems. Problem Set: As a future professional chemist, you will need to have good problem solving skills. You should also be able to use spreadsheet for data analysis. Tables and graphs are important part of analysis. Problem sets will give you opportunity to practice problem solving and critical thinking to explain various concepts. These problems will be more involved, and may need the use of spreadsheet. You are encouraged to work with your classmates. However, the answers should be prepared independently and not copied from others. Problem sets will usually be given on Wednesday and will be due on the following Wednesday at the beginning of the class. They will be graded and answer keys will be posted on Blackboard. No late problem set will be accepted. Problem Solving: The solution to each problem in exams, quizzes and problem sets must be written out clearly. It is easy to provide a feedback when work is shown clearly. Be sure to show all work leading to the final answer. Problems without full work will not be given any credit. Final answer must have correct unit. Points will be deducted for answers without correct unit. Regular problem solving and reading is essential for the success in this course. Cramming at last minute will not help. Tentative Class Schedule Date Sept 3 – Sept 24 Topic Chapter The properties of gases 1 The First Law of Thermodynamics 2 Exam I – Thursday, Sept. 25 Sept 26 - Oct 22 The Second Law of Thermodynamics 3 Physical transformations of pure substances 4 Exam II – Thursday, Oct. 23 Oct 24 – Nov 19 Simple mixtures 5 Phase diagrams 6 Chemical equilibrium - Electrochemistry 7 Exam III – Thursday, Nov. 20 Nov 21- Dec 12 Molecules in motion 21 The rates of chemical reactions 22 The kinetics of complex reactions 23 Final Exam (cumulative) - Friday, Dec. 19 Biochemistry Course Syllabus (BI302 or CH302) Spring, 2009 Biochemistry uses the principles and language of chemistry to understand biology at the molecular level. Place: Boyd 219 (class) and Boyd 109 (lab) Time: MWF 11:15-12:05 Registration and credit: Students may register for this course as either CH320 or BI320. The course is 4 credits and includes a lab. Students enrolled in this course should have completed General Chemistry and at least one semester of Organic Chemistry. Course Description: A study of the molecular basis of biological processes. Topics include the major classes of biological macromolecules, bioenergetics, catalysis and regulatory control, and metabolism with an emphasis on catabolic processes. Instructor: ` Dr. Susan Swope Office: Boyd 110 Office Hours: M 2-3; T 11-12; W 9-10 ; F 9-10. Phone: 535-2481 Text: Biochemistry, 6th edition by Berg, Tymoczko and Stryer, Freeman, 2007. Tentative Schedule: Date Topics Text Chapter Part I. Introduction Week 1 Biochemistry: An Evolving Science 1 Part II. Proteins and Nucleic Acids Week 1 Protein composition and function 2 Week 2 Exploring proteins and proteomes 3 Week 3 RNA, DNA and flow of genetic information 4 Week 4 Exploring Genes and Genomes 5 Hour Exam 1 Feb 25 Part III. Catalysis and Regulation Week 5 Hemoglobin: Portrait of a Protein in Action 7 Week 6-7 Enzymes: Basic Concepts and Kinetics Catalytic Strategies Regulatory Strategies 8 9 10 Part IV. Carbohydrates, Lipids and Membranes Week 8-10 Hour Exam 2 Carbohydrates Lipids and Cell Membranes Membrane Channels and Pumps Signal-Transduction Pathways 11 12 13 14 April 10 Part V. Metabolism Week 11 Metabolism: Basic Concepts and Design 15 Week 12 Glycolysis 16 Week 13 Citric Acid Cycle 17 Week 14 Oxidative Phosphorylation 18 Hour Exam 3 (Final Exam) Monday, May 11 at 11am See Web CT for detailed calendar, assignments, and class outlines. Grading: Problem Sets: Hour Exams: Lab: Total: 400 points (10 @ 40 points each) 600 points (3 @ 200 points each) 400 points (half of total points earned) 1400 points. Final Grade (%): (Total points earned/1400) X 100 Biochemistry Lab Schedule (Subject to Change) Spring, 2009 Date Topic Jan 29 Feb 5 Exercise 1: Preparing Solutions in Biochemistry Lab Exercise 2: Introduction to Spectrophotometry Feb 12 Experiment #1: Microburger Biochemistry: Extraction and Spectral Characterization of Myoglobin from Hamburger Feb 19 Experiment #2: Isolation and Purification of Yeast Alcohol Dehydrogenase (YADH) Using Cibacron Blue Chromatography Feb 26 Mar 5 Mar 12 Mar 26 Experiment #3: Kinetic Analysis of Yeast Alcohol Dehydrogenase Apr 7 Apr 14 Experiment #4 : DNA Profiling of the D1S80 Locus: A Forensic Analysis OR Experiment #4a: DNA Composition by HighPerformance Liquid Chromatography Apr 21 Apr 28 Experiment #5: Spectroscopic Measurement of the Redox Potential of Cytochrome c May 5 Lab #5 and Lab notebook due. Evaluation: Lab Exercise 1: Lab Exercise 2: Experiment 1: Experiment 2: Experiment 3: Experiment 4: Experiment 5: Lab notebook: 20 points 20 points 40 points 80 points 40 points 80 points 40 points 80 points Total: 400 points